Short Communication - (2022) Volume 11, Issue 5
Bioassay-guided fractionation of a methanol extract of the seeds of Juniperus przewalskii Komarov led to the isolation of one diterpene (3α-hinokiol). The structure was determined by means of 1D and 2D NMR spectroscopy, resulting in complete and unambiguous 1H and 13C NMR chemical shift assignments. This compound was evaluated for antiproliferative activities, and was demonstrated to exert significant cell growth inhibitory activity against human ovarian cancer (HO-8910) cells.
Juniperus przewalskii • Diterpene • Antitumour activity
Juniperus przewalskii Komarov Is a perennial tree distributed in Sichuan, Gansu, and Qinghai provinces of China. As an endemic and dominant species to the Qinghai-Tibet Plateau, J. przewalskii plays a key role in the maintenance of ecological balance in this region [1]. It belongs to the genus Juniperus in the family Cupressaceae, and the trees can grow to a height of up to 12 m. The lifespan of J. przewalskii can more than 3,000 years, and it grows on sunny side at an elevation gradient (about 2,500 m-4,000 m). The flowering period of J. przewalskii occurs relatively late (from June to July) and last for a relatively long time (15-25 d), and the trees begin to bear fruit at the age of approximately 15 years [2]. The essential oils of J. przewalskii are widely applied in food, medicine, and agriculture and forestry [3]. Addition, J. przewalskii is a Chinese and Tibetan medicine for the treatment of acute uterine bleeding, nephritis, and arthritis [4].
J. przewalskii is rich in essential oils, and these volatile compounds belong to phenols, terpenoids, and flavonoids [5]. They mainly include D-limonene, myrcene, terpinolene, citronellol, γ-terpineol, (-)-4-terpineol, α-juniperol, 4(10)-arborvitae, 13-hydroxy labda-8(17), 14-diene-19-ald, 4-hydroxyperoxide- 13-hydroxy-19-norla-bda-8(17), 14-diene, 4-epi-hydroxyperoxide-13-hydroxy- 19-norlabda-8(17), 14-diene, 19-acetoxy-13-hydroxylabda-8(17), 14-diene, α-pinene, 1-methyl-4-(1-methylethyl)-1, 4-cyclohexadiene, 4-methyl-1-(1- methylet-hyl)-3-cycohexen-1-ol, (s-(E, E))-1-methy-l-5-methylene-8-(1- methylethyl)-1, 6-cyclodecadiene, (1S-cis)-1, 2, 3, 5, 6, 8a-hexah-y dro-4, 7-dimethyl-1-(1-methylethyl)-naphthalene, 1R-(1. alpha., 3. alpha., 4.beta.)- 4-ethenyl, alpha,alpha-4-trimethyl-3-(1-methylethenyl)-cyclohexanemethanol, cedrol, β-phellandrene, thujopsene, (+) -a-muurolene, 4-methylene-1-(1- methylethyl)-bicyclo (3. 1. 0) hexane, caryophyllene, biocyclo (3. 1. 0) hex-2- ene, 4-methyl-1-(1-methylethyl)-, limonene, 1, 4-cyclohexadiene, 1-methyl- 4-(1-methylethyl)-, cyclohexe-ne, 1-methyl-4-(1-methylethylidene)-, 1, 6-octadien-3-ol, 3, 7-dimethyl-, 3-cyclohex-en-1-ol, 4-methyl-1-(1-methylethy)-, cyclohexane, 1-ethenyl-1-methyl-2, 4-bis (1-m-ethylethenyl)-, (1S-(1. alpha., 2. beta., 4. beta.))-, D1, 6-cyclodecadiene, 1-methyl-5-methylene-8-(1- methylethyl)-, (s-(E, E))-, naphthalene, 3α-hinokiol, propoxy-8-ced-rane, alphafunebrene, trans-totarol, naphthalene, ferruginol, sugiol, etc. In these volatile oils, one diterpenoid compound (3α-hinokiol) was found that it had strong antitumor activity against human ovarian cancer (HO-8910) cell lines [6].
Structure and bioassay
3α-hinokiol of the complete 1H NMR and 13C NMR determination assignments as listed in Table 1, and the chemical structure was exhibited in Figure 1. Cytotoxic effect was measured in vitro on the HO-8910 (human ovarian cancer) cell lines by using the MTT colorimetric assay, and vincristine was used as a positive control. 3α-hinokiol and vincristine demonstrated the similar activity with IC50 values of 63.1 μg/mL and 67.4 μg/mL on the HO-8910 cell lines, respectively [6]. The results showed that 3α-hinokiol had obvious inhibitory effect on this tumor cells.
| Order | 1H (m) | 13C (DEPT) |
|---|---|---|
| 1 | 1.05 (ddd, 11.3, 8.0, 5.0) 1.93 (dt, 11.3, 4.0) | 31.6 CH2 |
| 2 | 2.09 (m) 1.86 (m) | 25.9 CH2 |
| 3 | 3.49 (t, 2.8) | 75.8 CH |
| 4 | — | 37.5 C |
| 5 | 1.74 (dd, 11.0, 4.0) | 43.6 CH |
| 6 | 1.77 (m) 1.77 (m) | 18.8 CH2 |
| 7 | 2.78 (ddd, 12.8, 10.3, 4.0) 2.85 (ddd, 12.8, 5.6, 1.5) | 29.5 CH2 |
| 8 | — | 127.2 C |
| 9 | — | 148.3 C |
| 10 | — | 37.7 C |
| 11 | 6.63 (s) | 110.9 CH |
| 12 | — | 150.7 C |
| 13 | — | 131.5 C |
| 14 | 6.83 (s) | 126.6 CH |
| 15 | 3.10 (qq, 8.0) | 26.8 CH |
| 16 | 1.23 (d, 8.0) | 22.5 CH3 |
| 17 | 1.23 (d, 8.0) | 22.8 CH3 |
| 18 | 0.95 (s) | 22.1 CH3 |
| 19 | 1.03 (s) | 28.1 CH3 |
| 20 | 1.19 (s) | 24.6 CH3 |
| Note: 40 MHz 1H NMR, 100 MHz 13C NMR (CDCl3, TMS, W) | ||
Table 1. 1H NMR and 13C NMR of 3a-hinokiol.
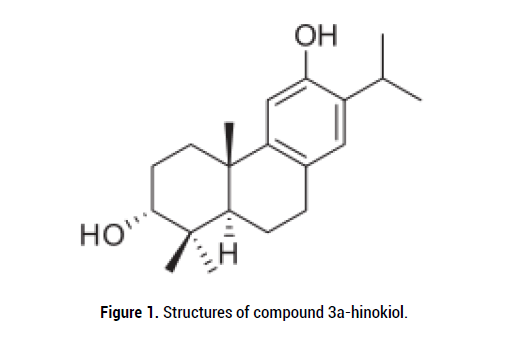
Figure 1: Structures of compound 3a-hinokiol.
One diterpenoid was isolated from the seeds of J. przewalskii and its structure was identified by the spectroscopic data. Compound 3α-hinokiol showed in vitro cytotoxicity against human ovarian cancer (HO-8910) cell lines. This detailed information is beneficial in the prevention and therapy of human tumors and other related diseases.
Citation: Zhang H, et al. 3?-Hinokiol with Antitumor Activity from Juniperus Przewalskii. J Biol Today's World, 2022, 11(5), 001-002
Received: 06-Sep-2022, Manuscript No. JBTW-22-73815; Editor assigned: 09-Sep-2022, Pre QC No. JBTW-22-73815 (QC); Reviewed: 23-Sep-2022, QC No. JBTW-22-73815; Revised: 30-Sep-2022, Manuscript No. JBTW-22-73815 (R); Published: 10-Oct-2022, DOI: 10.35248/2322-3308.11.5.004
Copyright: © 2022 Zhan H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.