Review Article - (2020) Volume 9, Issue 3
Discovering a new drug and developing it to the clinic is a, time-consuming and expensive process. A successfully developed patented drug enjoys market exclusivity which provides an opportunity to recoup the R&D expenses. Market competition with similar drugs from other companies, patent cliff and entry of generics present a threat to revenue generation. Successful pharma companies employ suitable Life Cycle Management (LCM) strategies, timed to expand the clinical utility of the drug and boost the revenues through new market exclusivities. Drug combination is one such strategy adopted to add therapeutic value and enhance the commercial life of a drug. In this article, we review drug combination as a Life Cycle Management (LCM) strategy adopted for the US Food and Drug Administration (FDA) approved drugs. We analysed New Molecular Entity (NME) approved by the FDA in the decade of 2001-2010 in four therapeutic areas of oncology, central nervous system, alimentary canal and metabolism and cardiovascular. The life cycle of these NMEs was tracked till 2019 focusing on fixed dose and free dose combination. We present case studies on sitagliptin and everolimus belonging to therapeutic areas metabolism and oncology, respectively, and critically discuss the life cycle of these two drugs. Through the presentation of the clinical utility and revenue generated for the drug combinations, we provide insights into drug combination as a life cycle management strategy.
Drug combination, LCM, Fixed dose, Free dose, Drug development.
LCM: Life Cycle Management; FDA: Food and Drug Administration; NME: New Molecular Entity; FDC: Fixed Dose Combination; FrDC: Free Dose Combination; API: Active Pharmaceutical Ingredient; NDA: New Drug Application; CNS: Central Nervous System; CVD: Cardiovascular Disease; T2D: Type-2 Diabetes; GLP-1: Glucagon-Like Peptide-1; HR: Hormone Receptor; HER2: Human Epidermal Growth Factor Receptor 2
Development and approval of a new molecular entity is a risky, expensive and time taking endeavour [1]. Various strategies are employed by pharma companies to enhance the utility of an approved drug and recoup the R&D expenses on the drug. Drug combination is an innovative life cycle management strategy through which patients and the drug developers benefit [2]. Combination strategy aims at expanding the therapeutic use of the drug for new indications and the existing patient populations or increasing the efficacy within a patient segment [3,4]. Drug combinations include fixed dose combination (FDC) or free dose combination (FrDC). FDC product is a single product containing two or more drug ingredients with all the ingredients contributing towards product’s effectiveness [5,6]. During the clinical development of FDC, some phases of clinical trials can be exempted if proper justification is provided [7]. Some of the advantages of FDC include an increase in efficacy, adherence and convenience for patients [5,8]. Reduction in pill burden is one of the major factors in therapeutic compliance of patients toward FDC [9,10]. However, FDC may not be advantageous for patients having adequate adherence to FrDCs [11]. Drugs can also be combined as free dose combination where multiple drugs are administered separately instead of a single pill having multiple active pharmaceutical ingredients (APIs) [12]. FrDCs may target an already approved indication or a newer patient segment within the approved indication, a newer indication or even an indication within an entirely different therapeutic area. FrDC provides the flexibility to alter the prescribed dose of a drug, if a side effect is seen. These advantages provide a rationale for the commercial success of combination therapy over monotherapy.
We collected all the NMEs approved by FDA during the decade starting from 2001 and ending in 2010 (method described below). We analyzed drug combinations associated with these NMEs in four therapeutic areas starting from their first approval. We present the FDCs and FrDCs for NMEs from four therapeutic areas, namely oncology, central nervous system (CNS), alimentary canal and metabolism, and cardiovascular disease (CVD). In addition, we present case studies on the life cycle of sitagliptin and everolimus, belonging to metabolism and oncology therapeutic areas, respectively, with a focus on combination strategy.
The USFDA Orange book database consists of information of API names, market status, application number, dosage form, route of administration, strength, date of approval, and applicant information [13]. The NMEs approved and enlisted for the decade 2001-2010, were captured from the USFDA Orange book. The NMEs were mapped to their ATC codes up to third description level based on WHO Collaborating Centre for Drug Statistics Methodology website (https://www.whocc.no/atc_ddd_index/) to further segregate them into different therapeutic classes. We classified the NMEs into different therapeutic categories. In this manuscript we have considered the NMEs belonging to only four therapeutic classes (i) oncology (ii) CNS (iii) alimentary tract and metabolism and (iv) CVD. These are among the top therapeutic areas in which maximum first-time drug approvals were obtained. These NMEs were analyzed for FDC and FrDC combinations approved till 2019. We selected sitagliptin and everolimus, belonging to metabolism and oncology therapeutic areas, respectively and followed their life cycle to provide insights.
A total of 102 NMEs were approved during 2001 to 2010 in these four therapeutic areas. A therapeutic area wise distribution of these NMEs is shown in Figure 1. The FDCs were obtained for NMEs within CVD (total 4 NMEs with 10 FDC products), alimentary canal and metabolism (total 4 NMEs with 8 FDC products), and CNS (1 NME with 1 FDC product), however there was no NME with an FDC in oncology (Table 1). In the case of FrDC, Oncology had the highest number of NMEs (total 17) with FrDCs, while CNS and alimentary canal and metabolism had 9 NMEs and CVD had 8 NMEs with FrDCs (Tables 2 and 3). All these FDC and FrDCs associated with the NMEs belonging to the four therapeutic areas were analyzed to select two NMEs for life cycle management case studies. The selected NMEs are sitagliptin, which mainly shows FDC-based products during its life cycle, and everolimus, which shows only FrDC to manage its life cycle.
| Therapeutic area | NMEs with FDC | NME approval year | Combinations |
|---|---|---|---|
| Oncology | None | None | |
| CNS | Memantine hydrochloride | 2003 | Memantine hydrochloride+Donepezil hydrochloride |
| Alimentary tract and metabolism | Saxagliptin hydrochloride | 2009 | Saxagliptin hydrochloride+Dapagliflozin | Saxagliptin hydrochloride+Metformin hydrochloride |
| Sitagliptin phosphate | 2006 | Sitagliptin phosphate+Ertugliflozi | Sitagliptin phosphate+Metformin hydrochloride | Sitagliptin phosphate+Simvastatin | |
| Palonosetron hydrochloride | 2003 | Palonosetron hydrochloride+Fosnetupitant chloride hydrochloride | Palonosetron hydrochloride+Netupitant | |
| Esomeprazole magnesium | 2001 | Esomeprazole magnesium+Naproxen | |
| CVD | Aliskiren hemifumarate | 2007 | Aliskiren hemifumarate+Amlodipine besylate | Aliskiren hemifumarate+Amlodipine besylate+Hydrochlorothiazide | Aliskiren hemifumarate+Hydrochlorothiazide | Aliskiren hemifumarate+Valsartan |
| Olmesartan medoxomil | 2002 | Olmesartan medoxomil+Amlodipine besylate+Hydrochlorothiazide | Olmesartan medoxomil+Amlodipine besylate | Olmesartan medoxomil+Hydrochlorothiazide | |
| Ezetimibe | 2002 | Ezetimibe+Atorvastatin calcium | Ezetimibe+Simvastatin | |
| Nebivolol hydrochloride | 2007 | Nebivolol hydrochloride+Valsartan |
Note: *Combinations are separated by ‘|’ and Drugs within a combination are separated by ‘+’
Table 1: Overview of the NMEs and FDCs approved in four therapeutic areas oncology, CNS, alimentary canal and metabolism, and CVD.
| Therapeutic area | NMEs with FrDC | NME approval year | Combinations |
|---|---|---|---|
| Oncology | Bortezomib | 2003 | Bortezomib+Melphalan+Prednisone | Bortezomib+Rituximab+Cyclophosphamide+Doxorubicin+Prednisone |
| Cabazitaxel | 2010 | Cabazitaxel+Prednisone | |
| Lenalidomide | 2005 | Lenalidomide+Dexamethasone | Lenalidomide+Rituximab | |
| Nilotinib hydrochloride | 2007 | Nilotinib hydrochloride+Erythropoietin | Nilotinib hydrochloride+Granulocyte-colony stimulating factor (G-CSF) | Nilotinib hydrochloride+Hydroxyurea | Nilotinib hydrochloride+Anagrelide | |
| Oxaliplatin | 2002 | Oxaliplatin+5-FU/LV+Irinotecan | Oxaliplatin+5-FU/LV | |
| Pemetrexed disodium | 2004 | Pemetrexed disodium+Cisplatin | Pemetrexed disodium+Carboplatin+Embrolizumab | |
| Plerixafor | 2008 | Plerixafor+G-CSF | |
| Pralatrexate | 2009 | Pralatrexate+Vitamin B12+Folic acid | |
| Temsirolimus | 2007 | Temsirolimus+Diphenhydramine | |
| Erlotinib hydrochloride | 2004 | Erlotinib hydrochloride+Gemcitabine | |
| Everolimus | 2009 | Everolimus+Exemestane | Everolimus+Basiliximab+Cyclosporine+Corticosteroids | Everolimus+Tacrolimus+Corticosteroids | |
| Fulvestrant | 2002 | Fulvestrant+Ribociclib | Fulvestrant+Palbociclib | Fulvestrant+Abemaciclib | |
| Ixabepilone | 2007 | Ixabepilone+Capecitabine | |
| Lapatinib ditosylate | 2007 | Lapatinib ditosylate+Capecitabine | Lapatinib ditosylate+Letrozole | |
| Azacitidine | 2004 | Azacitidine+Medication for nausea and vomiting | |
| Decitabine | 2006 | Decitabine+Medication for nausea and vomiting | |
| Imatinib mesylate | 2001 | Imatinib mesylate+Chemotherapy | |
| CNS | Aripiprazole | 2002 | Aripiprazole+Antidepressant | Aripiprazole+lithium | Aripiprazole+Valproate |
| Asenapine maleate | 2009 | Asenapine maleate+Lithium | Asenapine maleate+Valproate | |
| Lacosamide | 2008 | Adjuvant therapy | |
| Lurasidone hydrochloride | 2010 | Lurasidone hydrochloride+Lithium | Lurasidone hydrochloride+Valproate | |
| Ziprasidone hydrochloride | 2001 | Ziprasidone hydrochloride+Lithium | Ziprasidone hydrochloride+Valproate | |
| Paliperidone | 2006 | Paliperidone+Mood stabilizers+Antidepressants | Paliperidone+Mood stabilizers | Paliperidone+Antidepressants | |
| Pregabalin | 2004 | Adjuvant Therapy | |
| Rasagiline mesylate | 2006 | Rasagiline mesylate+Levodopa | |
| Rufinamide | 2008 | Adjuvant therapy |
Note: *Combinations are separated by ‘|’ and Drugs within a combination are separated by ‘+’
Table 2: Overview of the NMEs and FrDCs approved in therapeutic areas oncology and CNS.
| Therapeutic area | NMEs with FrDC | NME approval year | Combinations |
|---|---|---|---|
| Alimentary tract and metabolism | Aprepitant | 2003 | Aprepitant+Antiemetic agents | Aprepitant+Cisplatin+Dexamethasone+Ondansetron | Aprepitant+Dexamethasone+Ondansetron | Aprepitant+Dexamethasone+Ondansetron+High-dose cisplatin | Aprepitant+Chemotherapy+Dexamethasone+Ondansetron | Aprepitant+High-dose cisplatin+Dexamethasone+Corticosteroid+5-HT3 antagonist | Aprepitant+Chemotherapy+Dexamethasone+Corticosteroid+5-HT3 antagonist |
| Pramlintide acetate | 2005 | Pramlintide acetate+Insulin | |
| Sitagliptin phosphate | 2006 | Sitagliptin phosphate+Metformin | Sitagliptin phosphate+Peroxisome proliferator activated receptor gamma (PPARγ) agonist | |
| Exenatide synthetic | 2005 | Exenatide+Metformin | Exenatide+Sulfonylurea | Exenatide+Metformin+Sulfonylurea | |
| Fosaprepitant dimeglumine | 2008 | Fosaprepitant dimeglumine+Cisplatin+Dexamethasone+Ondansetron | Fosaprepitant dimeglumine+Dexamethasone+Ondansetron | |
| L-glutamine | 2004 | L-Glutamine+Somatotropin | |
| Liraglutide recombinant | 2010 | Liraglutide recombinant+Sulfonylureas | Liraglutide recombinant+Insulin | |
| Omeprazole magnesium | 2003 | Omeprazole magnesium+Amoxicillin+Clarithromycin | Omeprazole magnesium+Amoxicillin | Omeprazole magnesium+Clarithromycin | |
| Granisetron | 2008 | Granisetron+Chemotherapy | Granisetron+Anthracycline+Cyclophosphamide+Dexamethasone+NK1 receptor antagonist | |
| CVD | Aliskiren hemifumarate | 2007 | Aliskiren hemifumarate+Antihypertensive |
| Ambrisentan | 2007 | Ambrisentan+Tadalafil | |
| Nebivolol hydrochloride | 2007 | Nebivolol hydrochloride+ACE Inhibitors+Angiotensin II receptor antagonists+Thiazide diuretics | |
| Olmesartan medoxomil | 2002 | Olmesartan medoxomil+Antihypertensive | |
| Eplerenone | 2002 | Eplerenone+Antihypertensive | |
| Ezetimibe | 2002 | Ezetimibe+HMG-CoA reductase inhibitor | Ezetimibe+Atrovastatin | Ezetimibe+Simavastatin | Ezetimibe+Lipid altering agents | |
| Ranolazine | 2006 | Ranolazine+Amlodipine+Beta-blockers+Nitrates | |
| Rosuvastatin calcium | 2003 | Rosuvastatin calcium+Lipid altering agents |
Note: *Combinations are separated by ‘|’ and Drugs within a combination are separated by ‘+’
Table 3: Overview of the NMEs and FrDCs approved in therapeutic areas alimentary canal and metabolism, and CVD.
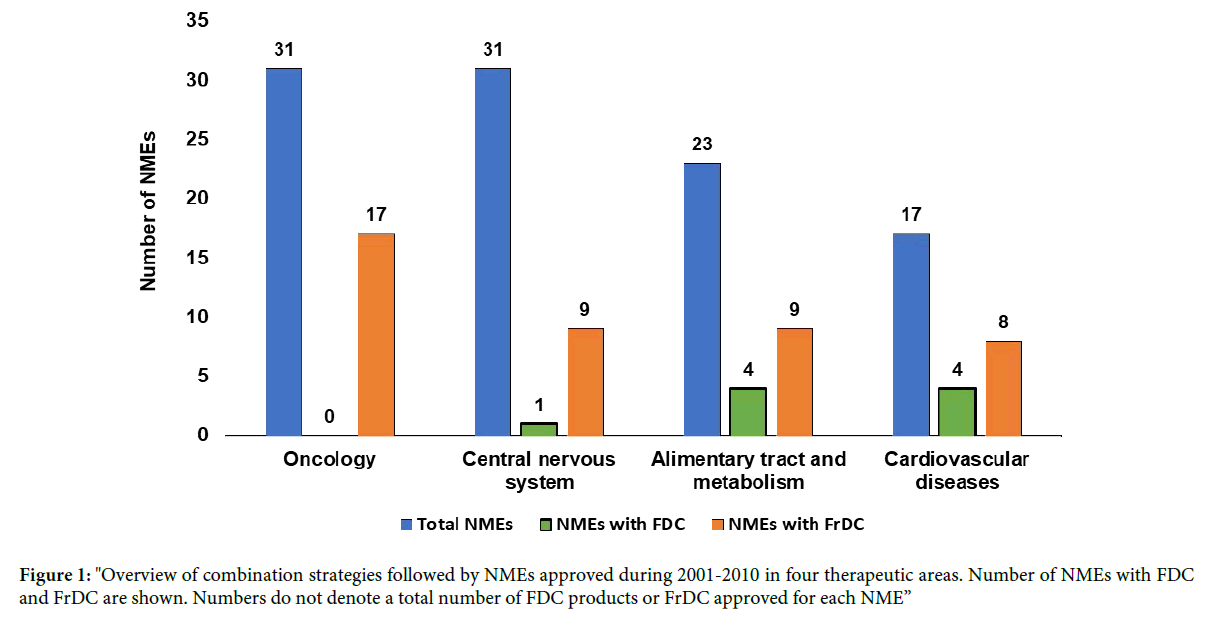
Figure 1: "Overview of combination strategies followed by NMEs approved during 2001-2010 in four therapeutic areas. Number of NMEs with FDC and FrDC are shown. Numbers do not denote a total number of FDC products or FrDC approved for each NME”
Fixed dose combination strategy positively modulates exclusivity profile of a drug
Despite various attempts made by the innovator company to extend the exclusivity of the NME, the entry of generic drug is inevitable. These generic drugs are copies of the original NMEs with original dose, formulation and therapeutic use (indication) [14]. The entry of generic drug into the market causes sales drop (of an NME) by more than 60% within a year [15]. To emphasize the importance of FDC as a strategy to improve the life cycle of a drug, we have chosen the case study of sitagliptin phosphate where the FDCs improved its life cycle.
Sitagliptin case study-an FDC product-based life cycle management: In the therapeutic area of alimentary tract and metabolism, we analyzed all the FDCs in the study period (Table 1) and shortlisted sitagliptin phosphate as a fit case study to showcase the successful implementation of FDC as an LCM strategy. We looked at the approval landscape of sitagliptin phosphate whose generics have not been approved yet. On October 16, 2006, Merck’s sitagliptin phosphate (Januvia; oral Tablet) received the FDA approval as a supplementary treatment to diet and exercise for improving glycemic control in patients with diabetes type-2 (T2D) [16]. Sitagliptin phosphate inhibits dipeptidyl peptidase 4, protecting Glucagon-like peptide-1 (GLP-1) from degradation. GLP-1 plays an important role in glucose homeostasis [17] and is critical for maintaining the normoglycemia. The following year, in 2007, the FDC product of sitagliptin phosphate, Janumet (sitagliptin phosphate+metformin hydrochloride), was approved (Table 1). Janumet is indicated for patients with T2D whose blood glucose levels are not sufficiently controlled on either Metformin or sitagliptin monotherapy (Figure 2). The FDC strategy, thus, filled the therapeutic gap for patients unresponsive to monotherapy and is a case in point for a patient segment with unmet medical need [18]. A look at the revenue profile of sitagliptin monotherapy (Januvia), sitagliptin FDC product Janumet and their combined revenue (Figure 3) clearly shows that the fixed dose combination significantly contributed to the total revenue from sitagliptin [19].
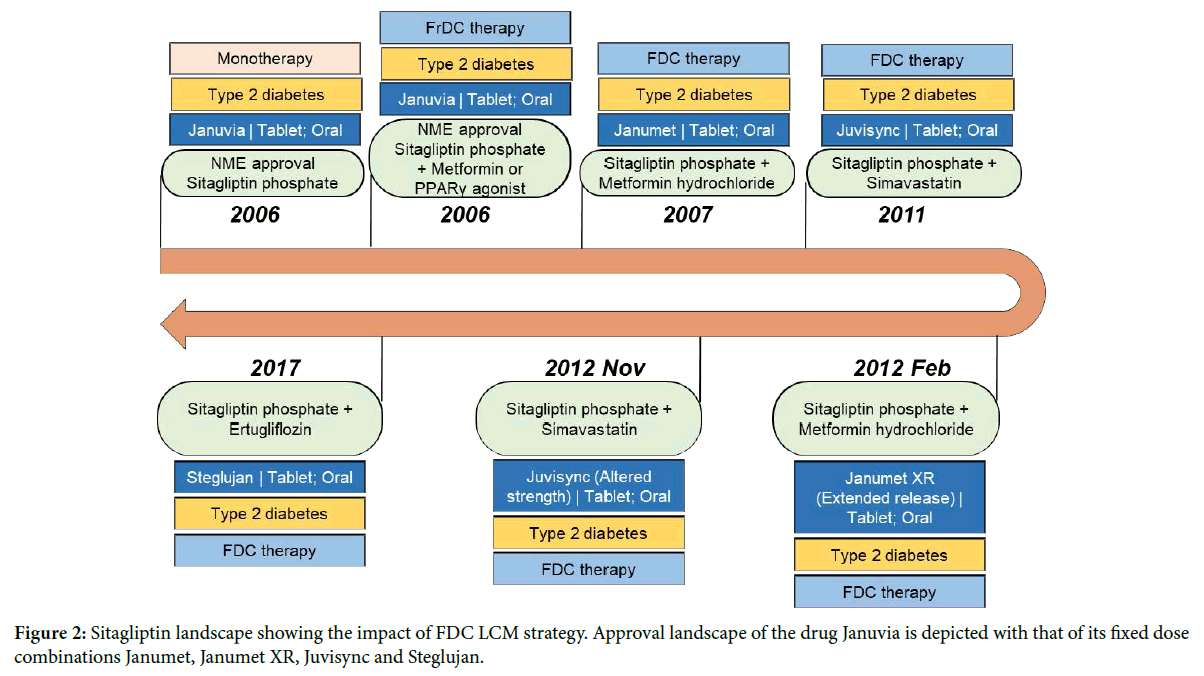
Figure 2: Sitagliptin landscape showing the impact of FDC LCM strategy. Approval landscape of the drug Januvia is depicted with that of its fixed dose combinations Janumet, Janumet XR, Juvisync and Steglujan.
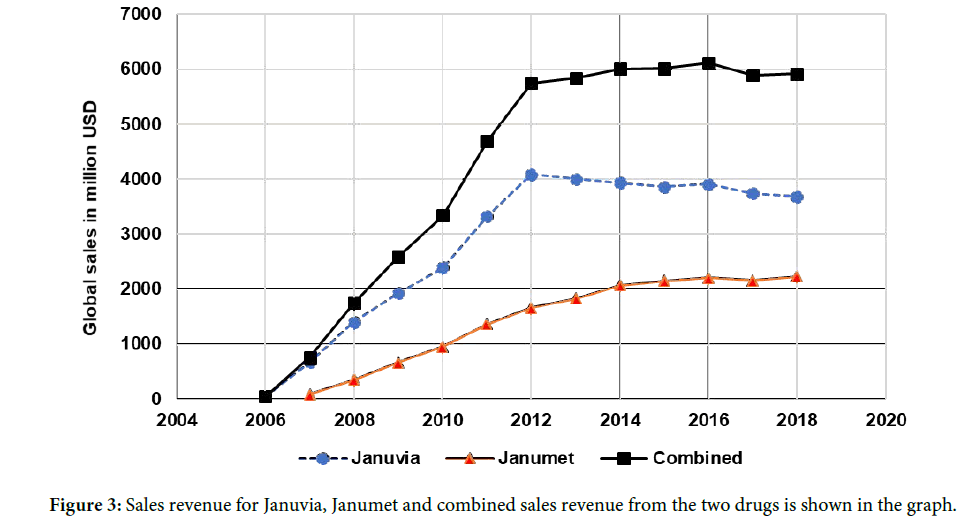
Figure 3: Sales revenue for Januvia, Janumet and combined sales revenue from the two drugs is shown in the graph.
In 2011, Merck also got the FDA approval for its second FDC with sitagliptin phosphate i.e. Juvisync (sitagliptin+simvastatin), a novel treatment for T2D that combines the glucose lowering medication with the cholesterol-lowering medication, simvastatin. In February 2012, Merck received the FDA approval for the reformulated Janumet with extended-release metformin giving a convenient once-daily treatment option for patients with T2D. In late 2012, Merck also released Juvisync with altered strength. However, in 2013, Merck voluntarily discontinued Juvisync for business related reasons.
Merck holds the drug product and drug substance patent for Januvia until July 26th, 2022 (patent number: 6699871). The approaching patent expiry of Januvia demanded a line extension strategy to reassure the market exclusivity to Merck. At least half a dozen companies received tentative approval for the generic forms of sitagliptin phosphate and are awaiting market entry. In 2017, Merck received the FDA approval for its third FDC product of sitagliptin phosphate, Steglujan. Steglujan is a combination of sitagliptin phosphate and ertugliflozin, an oral sodium-glucose cotransporter 2 inhibitor [20]. Merck, thus, extended the life of sitagliptin phosphate up to July 13th, 2030 as the patent period of Steglujan is up to 2030 (patent number: 8080580). Hence, sitagliptin can be considered as one of the classic cases where Merck successfully implemented the LCM strategy using FDC products to fill the therapeutic gap for the diabetic patients to earn revenue even after the expiry of sitagliptin exclusivity. The revenue details revealed that the Janumet (sitagliptin+metformin) has helped to boost the total revenues generated from sitagliptin (Figure 3).
Free dose combination as a strategy for drug life cycle management
FrDCs associated with the NMEs were analyzed for the study period as described in the methods. Out of the four therapeutic areas, oncology had the highest number of NMEs (total 17). CNS and alimentary canal and metabolism had 9 NMEs each with FrDC and CVD had 8 NMEs with FrDCs (Tables 2 and 3). The landscape of all the NMEs belonging to antineoplastic agents were analyzed for the associated FrDCs and the indications to which they have been approved by the FDA. An interesting case of everolimus life cycle management has been discussed here which underwent multiple indication expansions through FrDC strategy.
Everolimus case study – an FrDC-based life cycle management: Everolimus was approved in 2009 by the FDA as oral tablet (Afinitor) for the treatment of advanced renal cell carcinoma after sunitinib or sorafenib treatment failure [21]. Everolimus is a derivative of rapamycin that works like rapamycin. It functions as a mammalian target of rapamycin (mTOR) inhibitor. Novartis obtained an additional approval for different strengths of new form of dispersible tablet (Afinitor Disperz) in the year 2012. Besides the original indication of advanced renal cell carcinoma, Afinitor was also approved as a monotherapy to treat advanced neuroendocrine tumors, Renal angiomyolipoma with tuberous sclerosis complex, subependymal giant cell astrocytoma with tuberous sclerosis complex and tuberous sclerosis complexassociated partial onset seizures (Figure 4).
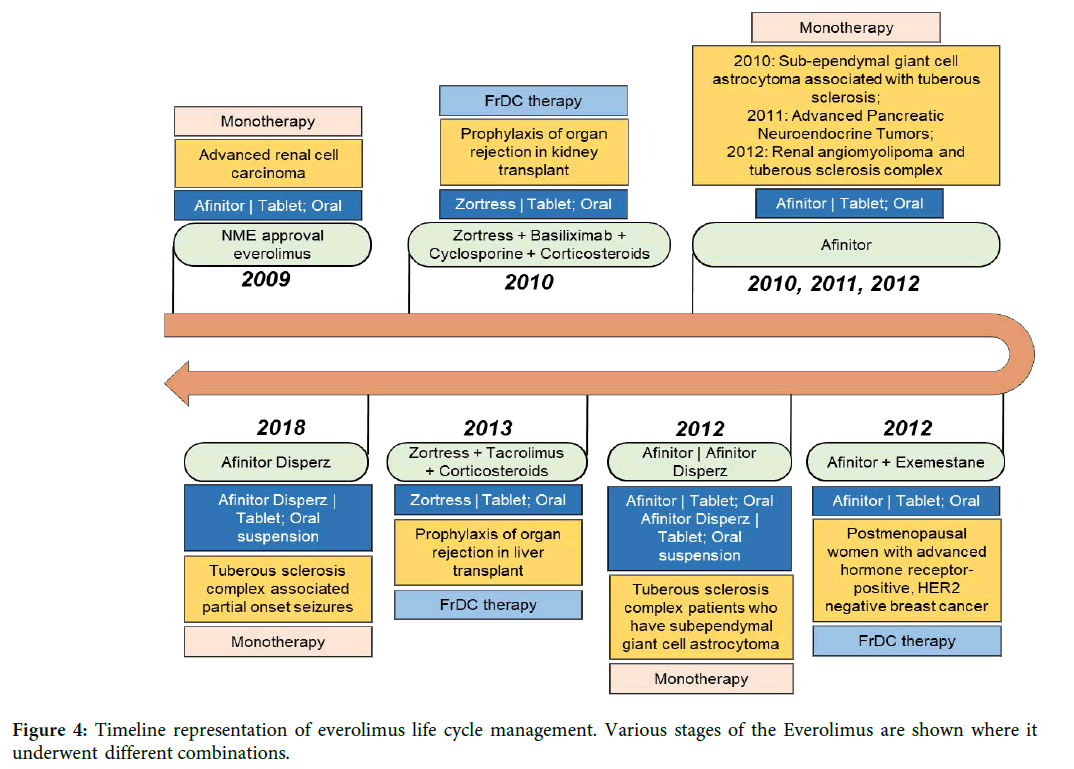
Figure 4: Timeline representation of everolimus life cycle management. Various stages of the Everolimus are shown where it underwent different combinations.
In an FrDC with exemestane (Cytochrome P450 19 aromatase inhibitor), everolimus was approved for HR receptor positive (HR+) and HER2-negative (HER2-) advanced breast cancer in postmenopausal women after the failure of treatment with letrozole or anastrozole. Interestingly, Novartis also got approval for everolimus with a different brand name, Zortress for the treatment of prophylaxis of organ rejection in kidney transplant in combination with basiliximab, cyclosporine and corticosteroids. In 2013, Zortress was also approved for prophylaxis of organ rejection in liver transplant in combination with tacrolimus and corticosteroids [22]. Clearly, combination strategy has resulted in expanding the therapeutic utility of everolimus into a completely different therapeutic domain (Liver and kidney transplant rejection) from where everolimus monotherapy was originally approved (advanced renal cell carcinoma). Annual global sales data from Novartis shows that Zortress sales enhances the revenue of Afinitor (Figure 5) [23]. Also, with the FrDC strategy, Novartis obtained the exclusivity for the treatment of newer diseases belonging to same or different therapeutic area and extend the life of its drug everolimus. In April 2018, generic version of Zortress has been approved by the FDA (Application number: 206133). Generic versions of Afinitor Disperz and Afinitor were also approved in April and December 2019 respectively. The entry of the generic form of Zortress in 2018 did not impact the revenue of Novartis innovator drug (Figure 5).
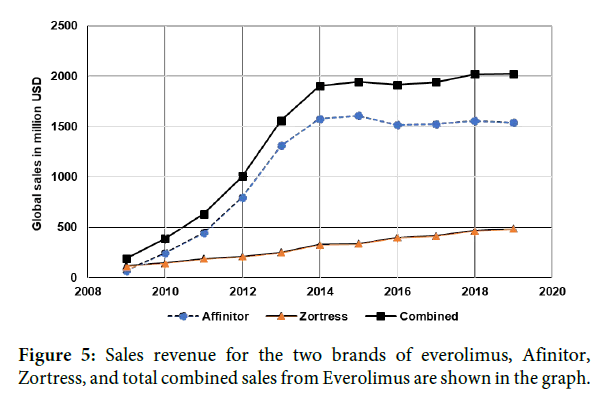
Figure 5: Sales revenue for the two brands of everolimus, Afinitor, Zortress, and total combined sales from Everolimus are shown in the graph.
This study analyzed FDC and FrDC drugs where at least one of the API in combination is FDA approved as NME during 2001-2010 in the four therapeutic areas. Majority of the FDCs are employed by CVD and alimentary canal and metabolism therapeutic area. In oncology, there are no FDCs observed probably because of the nature of the treatment as well as the disease. However, we could see a greater number of the FrDCs in oncology compared to other therapeutic area as the cancer treatment requires different drugs with varying doses.
In the case of sitagliptin life cycle, the FDC product Janumet not only contributed to the total revenue but also filled the therapeutic gap for patients unresponsive to monotherapy with either sitagliptin or metformin. Reformulated product of Janumet (extended release) is an example of an FDC product where patient convenience and adherence are addressed [18]. Juvisync, a combination of sitagliptin and simavastatin addresses a comorbidity of T2D, high blood cholesterol levels [24]. Launching of third sitagliptin FDC product Steglujan extended the life cycle of sitagliptin through market exclusivity. These LCM strategies for sitagliptin highlight the importance of FDC in therapeutic gap filling and revenue management.
In the case of everolimus case study, the FrDC of everolimus with exemestane lead to therapeutic use of everolimus in a completely different indication within the same therapeutic area. Two other FrDCs for everolimus are indicated for an entirely different disease area i.e., organ transplant rejections. Additionally, Novartis rebranded everolimus (Afinitor) to Zortress for its use in organ transplant rejection as FrDCs. Creating a new brand Zortress allows improved marketing and is used as a strategy to connect better with both the physician and the patients of completely different therapeutic area.
Overall, combination strategy allows a better market access and is an effective way of life cycle management.
The strategy to use FDCs is practiced to a varying degree in different therapeutic areas. However, due to the nature of treatment required for oncology diseases, FDC is not a choice of combination strategy. FDC products of sitagliptin provide value in filling the therapeutic gap, addressing comorbidities, improving patient convenience and the revenue management. The FrDC strategy used for everolimus resulted in venturing not only into different indications in the same therapeutic area but also helped in entry into a different therapeutic area. Combination strategies may also be used in conjunction with other LCM strategies, such as reformulation, creating value as shown by sitagliptin combination with extended-release metformin. Collectively, these studies provide insights into how combination strategies can be used to optimally manage the drug life cycle and provide benefit to patient and boost sales revenue.
The authors wish to acknowledge the help of Kunal Dayma for the initial writeup.
S.S.J., D.P., and K.M.M. curated and compiled the data; P.R.R., K.M.M., S.S.J., and D.P. analyzed the data; P.R.R., S.S.J., D.P. and S.K.S. wrote the article; S.S.J. and S.K.S., internally reviewed the article; S.K.S., and N.G. conceived the article.
The authors declare that there is no conflict of interest of any kind for the publication of this article. There is no conflict of interest related to the financial benefit for the data presented in this article.
Received: 25-Feb-2020
Copyright: �?�?�?â??�?â??�?�© 2020 Rikkala PR, et al. This is an open access paper distributed under the Creative Commons Attribution License. Journal of Biology and Today's World is published by Lexis Publisher.