Mini Review - (2022) Volume 3, Issue 4
Supercritical water gasification is a promising and environmentally friendly biomass conversion method. Compared to conventional gasification, it is capable of handling biomass with high moisture and displays higher conversion efficiency and decreased tar formation. Through supercritical gasification, organic feedstock in the presence of supercritical water is converted into a mixture of H2 , CO2 , CO, CH4 and traces of other gases over shorts periods of time. The composition and yield of the final gas mixture depends highly on reaction temperature, feed concentration and nature of catalyst in use. Reaction pressure and residence time play a less important role. Despite being appealing for the production of hydrogen from an inexpensive source, hydrothermal gasification suffers from decisive technical challenges, with corrosion and efficiency being the most critical.
Liquefaction • Gasification • Thermochemical • Pyrolysis
Biomass poses as an attractive renewable energy source. It is practically abundant and does not participate in the increase of carbon dioxide in the atmosphere. However, the direct use of biomass as a fuel is challenging as it is a carrier of low energy density and possesses high moisture content. In addition, biomass displays heterogeneity, hydrophilic behavior and poor grindability. Conversion technologies that improve the physicochemical properties of the initial feedstock are necessary [1-3].
The conversion of biomass takes place through two main groups of processes: biochemical and thermochemical processes [4, 5]. Biochemical processes convert biomass into energy primarily through fermentation and anaerobic digestion. Fermentation leads to the production of bioethanol through the use of microorganisms and enzymes, while anaerobic digestion employs bacterial activity in an anaerobic environment for the production of biogas [4-6].
Thermochemical processes use heat under the presence of a catalyst. The main thermochemical pathways are combustion, liquefaction, gasification and pyrolysis [4, 6]. The direct combustion of biomass is the most technologically mature method even though it requires feedstock with low moisture and is characterized by low efficiency [4, 5, 7]. Pyrolysis takes place under elevated temperatures (between 500oC-1000oC ) in an oxygen-deprived environment and leads to the production of bio-oil (bio-crude), a liquid fraction with high heating value, biochar, a solid carbon-rich residue suitable for soil amendment, and a mixture of non-condensable gases [5, 8, 9]. In hydrothermal liquefaction, commonly known as liquefaction, biomass is being converted into bio-oil in the presence of both a solvent and a catalyst and at temperatures between 200oC-400oC [10-13]. Gasification involves the partial oxidization of biomass at high temperatures (700oC-1500oC) within a gasification medium (air, steam or oxygen). The main product is a mixture of hydrogen, carbon monoxide, carbon dioxide, methane, and other gases in smaller quantities [4, 14-16]. In Figure 1, the main thermochemical routes of biomass conversion are depicted [17].Thermochemical processes, when compared to biochemical, display specific advantages. While biochemical methods are biomass-specific, thermochemical are capable of using a wider variety of biomass types. Their duration spans from a few minutes to several hours, when biochemical require several days. Finally, thermochemical processes are characterized by higher conversion efficiency [4, 6, 9].
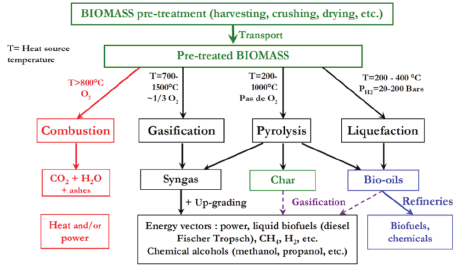
Figure 1: Main thermochemical routes for biomass conversion.
Most thermochemical processes are dry conversion processes, meaning that they can work efficiently only with organic feedstock that has low moisture content. Hydrothermal carbonization, liquefaction and gasification are capable of handling organic feedstock with high moisture content since the presence of water is required. Thus, the initial energy-intensive drying step is rendered redundant. In addition, the conditions (reaction temperature and pressure) affect the properties of water in ways that are beneficial to the degradation of biomass and its subsequent reformation into the final products [18-20]. In Figure 2, the classification of hydrothermal processes as a function of reaction temperature and pressure is shown.
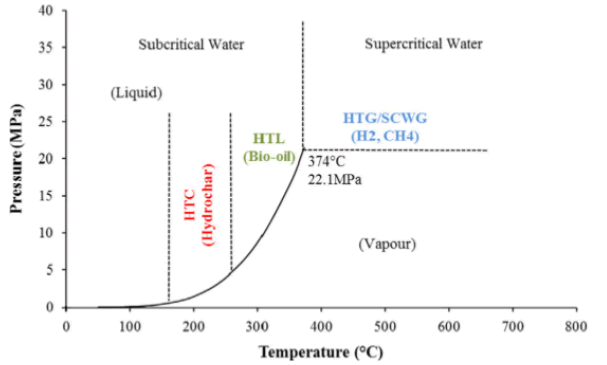
Figure 2: Hydrothermal processes as a function of temperature and pressure.
The focus of this paper is the process of supercritical water gasification. SCWG is a specific type of hydrothermal gasification that takes place under supercritical water conditions and leads to the production of hydrogen. Compared to its dry counterpart, it requires lower reaction temperatures, leads to lower tar formation, and, depending on the process parameters, may display relatively high conversion rates. Furthermore, hydrogen as a fuel is environmentally clean since its combustion releases water [19, 21-27].
Supercritical water gasification
Gasification is a thermochemical conversion that produces a combustible mixture of gases (mainly H2 , CO, CO2 and CH4 ) through the partial oxidization of solid organic feedstock. It takes place under high temperature (700oC-1500oC ) and pressure (20 MPa-30 MPa) and in the presence of a gasification agent such as air, steam or oxygen [14]. Hydrothermal gasification is a variation that involves lower temperatures and the use of water as a reaction medium. The aqueous environment makes the process ideal for the conversion of wet biomass with high moisture content [14, 21]. Hydrothermal gasification can be classified into three categories: catalyzed aqueous-phase reforming (215oC -265oC) for the production of hydrogen, catalyzed gasification (350oC -400oC) for the production of methane, and supercritical water gasification (600 oC-700 oC) for the production of hydrogen [18-23].
Supercritical Water Gasification (SCWG), which is the focus of this paper, displays specific advantages over conventional gasification. As a hydrothermal process, it takes place in the presence of water under high temperature and pressure (higher than 374 and 22.1 MPa)steam. The use of water as a reaction medium makes the process ideal for handling organic feedstock with high moisture content and at the same time renders the preliminary energy-intensive drying step of conventional gasification processes redundant. Furthermore, water is capable of dissolving intermediate products, thus reducing the formation of tar and solid by-products [18-24]. Under supercritical conditions, water acts both as a reaction medium and as a reactant. Its properties (density, dielectric constant, ion product) are beneficial to the degradation of organic compounds [28].
Supercritical water gasification mainly involves the following three reactions: (1) steam reforming, (2) water-gas shift and (3) methanation reactions.

The two-way arrow suggests that the respective reactions are in equilibrium and, depending on the process parameters, may proceed either way [21, 29]. In steam reforming, supercritical water reacts with organic feedstock producing hydrogen and carbon monoxide. The carbon monoxide reacts, in turn, with water producing hydrogen and carbon dioxide via water-gas shift reaction. In methanation reaction, carbon monoxide from steam reforming and hydrogen from water-gas shift react producing methane and water [15, 28, 30].
The conventional gasification technologies consist mainly from fixed bed, fluidized bed and entrained bed gasifiers (Figure 3). Fixed bed gasifiers are further classified into updraft and downdraft, depending on the direction of the airflow. In an updraft fixed bed gasifier the fuel inlet and gas outlet reside at the top while the inlet of the gasifying agent resides at the bottom. The rising gasifying agent comes in contact with hot unconverted biomass charcoal and hot CO2 and H2O are produced, which in turn react endothermically with char and form CO and H2 . Even thought updraft gasifiers have very high conversion efficiencies (up to 80%), the produced gases contain high amounts of tar. Downdraft gasifiers display lower efficiency since the gases exit at high temperatures (900oC-1000oC). In downdraft gasifiers, the biomass feed and the air are moving in the same direction even though air enters the rector at a lower part of the gasifier through radially directed nozzles. The fact that the gases pass through the hot zone before exiting, enables the partial cracking of formed tar leading to lower tar content. Downdraft gasifiers, when compared to updraft display lower efficiency since the gases exit at high temperature. On the other hand, the final product contains less amount of tar and ash.
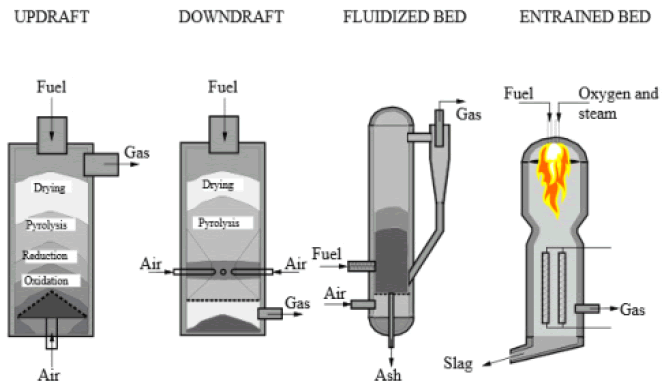
Figure 3: Conventional gasification technologies: fixed bed (updraft and downdraft, fluidized bed and entrained bed).
In the case of fluidized bed gasifiers, the air is moving upwards through a biomass bed playing the role of the fluidizing gas. This provides temperature uniformity since air and solid phase come in excellent thermal contact. Fluidized bed gasifiers employ temperatures between 800oC-1000oC in order to avoid the accumulation of ash. Despite its flexibility in terms of feed rate and consumption rate, these gasifiers produce more ash and tar compared to downdraft gasifiers. Entrained bed gasifiers encounter problems processing fibrous materials and therefore are unsuitable for many types of biomass and applications [14-16, 18, 19, 22, 31].
Process parameters
Reaction temperature and pressure, biomass concentration, residence time and the use of catalysts have been recognized as the most influential process parameters.
Temperature is considered to be the most dominant parameter that affects gasification efficiency and product quality. Higher temperatures favor H2 and CO2 formation, while lower temperatures are associated with increased CH4 and CO yields. This is attributed to the underlying reaction mechanism. At lower gasification temperatures, the endothermic methanation reactions are prevalent, while at higher temperatures the exothermic reforming and water-gas shift reactions are being promoted (Le Chatelier principle or equilibrium law). This is why temperatures up to 400℃ favor methane production, while higher temperatures up to 600℃ or higher lead to increased hydrogen yields [18, 32]. At higher temperatures, SCWG achieves higher product yield at the cost energy efficiency. The use of catalysts can effectively lower the gasification temperature [18, 32]. Reaction temperature also affects the production of CO and CO2 similarly. As temperature increases, CO2 production increases at the expense of CO [24].
It has been found that by increasing pressure the gas composition shifts in favor of methane. The effect of reaction pressure, however, was found to be negligible in comparison to the reaction temperature [24, 32].
Feed concentration has an adverse effect on gasification efficiency. Apart from being associated with pumping and plugging issues, high feedstock concentrations are linked with reduced hydrogen and increased methane yields. This is attributed to the amount of water required for the formation of each product. H2 requires much more water than CH4. An example of the effect of temperature and feed concentration on gas yields is depicted in Figure 4. The hydrogen and methane yields of wood sawdust gasification are evidently antagonizing [24, 32].
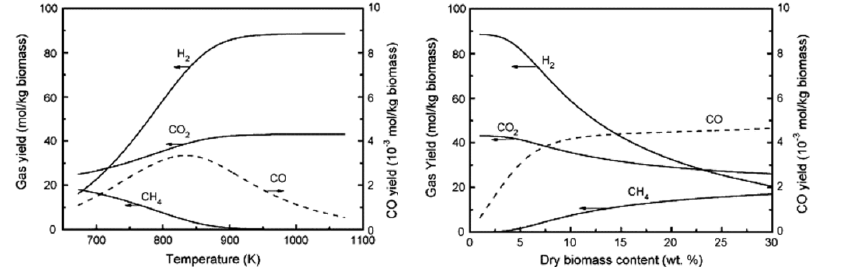
Figure 4: Gas yields as a function of temperature and dry biomass content of wood sawdust gasification.
Biomass decomposition generally occurs rapidly in SCWG. Residence time also affects the product yield but at a lesser extent. Initially, prolonged retention times lead to increased gas production. However, after a certain point, the effect of duration is marginal. Whether prolonged times will benefit methane or hydrogen yield depends on the feedstock in use [19, 21, 27].
SCWG takes place under high temperatures and pressure. The use of specific catalysts such as alkali, transition metals and activated carbon that improve the process has been investigated. The presence of a catalyst lowers the required extreme operating conditions, thus improving the efficiency of the process. In addition, catalysts are connected with improved H2 yields (high hydrogen production selectivity) and the suppression of tar and char formation [18, 27].
Homogeneous catalysts such as alkali metals (Na2CO3, KHCO3, K2CO3, NaOH etc) are often used to improve the water-gas shift reaction. Such catalysts, however, are dissolved in the supercritical water, making it difficult to recover. They are also associated with reactor fouling, plugging, and corrosion. Heterogeneous catalysts consist of transition metals (Ni, Ru, Rh, Pt etc), activated carbons, and oxides (oxides of Cu, Mn, Co, Al, and others). In addition, they display higher hydrogen selectivity and catalytic activity. Heterogeneous catalysts are considered superior to their homogeneous counterparts due to their increased recyclability. Metal catalysts enhance methanation and steam reforming reactions while alkali catalysts enhance water-gas shift reactions. Among the typical SCWG catalysts are KOH, KHCO3, Na2CO3, and the noble metals Ru, Rh and Pt [18, 23, 28, 32].
Technical challenges
Supercritical water gasification is considered to be a promising biomass conversion method. However, its commercial applications face several challenges. SCWG is a relatively expensive application and therefore is suitable for the conversion of biomass with high disposal cost. Due to the high cost of installation and operation, the integration of SCWG with other processes for power generation and CO2 capture has been speculated [21, 33]. Compared to other conventional methods for the production of hydrogen, supercritical water gasification is the most cost effective process. Compared to fossil fuels, the cost of hydrogen produced by renewable energy sources is still very high [30].
SCWG is often associated with plugging and corrosion of the reactor [21]. This is attributed to the extreme operating conditions and to unwanted intermediate products that are formed during the process. SCWG reactors require materials that are able to withstand these adverse conditions of temperature and pressure as well as the corrosion. Nickel-based alloys as reactor construction materials is a common solution. Another promising alternative which is currently under investigation is the use of ceramic materials such as aluminum oxide (alumina, Al2O3). Novel circulating flow reactor designs that prohibit the contact of corrosive materials with solid surfaces have also been proposed. Apart from increasing the selectivity to hydrogen and suppressing the formation of tar, the use of catalysts may be employed for the efficient reduction of the required reaction temperature and pressure [18, 27, 30, 34]. A research effort has been made towards the development of tubular reactor that deals with plugging. These reactors are prone to intermittent function due to the formation of char and ash build-up. It has been speculated that high reaction temperature and high heating rate at the entrance of the reactor may be beneficial to the plugging of the reactor [27, 35].
Even though supercritical water gasification does not require a preliminary feedstock drying, whose energy is irrecoverable, it still suffers from thermal efficiency issues. Fortunately, despite the high temperature requirement, a significant amount of sensible heat can be recovered from the exiting gaseous mixture using a high efficiency heat exchanger. This would increase the overall performance of the process [14, 27, 32, 33]. Finally, the feedstock composition may be of importance. Experiments have shown that lignin content has a negative effect on hydrogen production. Intermediate products react with lignin, thus reducing the hydrogen yield [24, 36].
Supercritical water gasification is a biomass conversion method that stands out. It is capable of converting organic feedstock with high moisture content into hydrogen and other gases with high yields over short times. High operating pressures allow less energy consumption for gas storage and smaller reactor dimension and consequent heat losses. Feedstock and reaction medium are inexpensive. Its final product is superior from that of conventional dry gasification. However, the application of SCWG at an industrial level faces important techno-economical obstacles. This conversion method is relatively new and a limited amount of applications exist outside a laboratory. Frequent maintenance and product purification are adding to the pre-existing installation complexity and high operating costs. The severe operating conditions, the issues of reactor corrosion and plugging are a challenge for the designing and manufacturing of gasification reactors. Understanding the underlying reaction mechanism combined with newer reactor designs and experimentation on catalysts will hopefully lead towards a more efficient hydrogen production.
Citation: Kladisios P, Sagia A. A Review on Supercritical Water Gasification of Biomass. Bioenergy Bioresour: Open Access. 2022, 3(4), 07-10
Received: 01-Jun-2022, Manuscript No. BBOA-22-65622; Editor assigned: 03-Jun-2022, Pre QC No. BBOA-22-65622 (PQ); Reviewed: 22-Jul-2022, QC No. BBOA-22-65622 (Q); Revised: 08-Aug-2022, Manuscript No. BBOA-22-65622 (R); Published: 18-Aug-2022, DOI: 10.37532/bboa.22.3.4.7-10
Copyright: © 2022 Kladisios P. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.