Case Report - (2020) Volume 9, Issue 5
Sickle cell anemia is an inherited hemoglobin disease caused by the substitution of valine for glutamic acid in position 6 of the ß chain of globin. It is the most common genetic disease in the world. Its severity is linked to its complications. Asplenia is a rare chronic complication due to repeated infarctions. The authors report a case of asplenia in a 12-year-old thalasso-sickle cell patient.
Asplenia, Sickle cell anemia, Child.
Sickle cell disease is an autosomal recessive inherited hemoglobin disease resulting from a point mutation of the sixth codon of the β globin gene located on chromosome 11 [1]. The major sickle cell syndromes include homozygous SS, double heterozygous composite SC, and sickle beta thalassemia [1]. According to the WHO, nearly 5% of the world population is carriers of a gene responsible for a hemoglobin abnormality [2]. The majority of people suffering from this disease live in Black Africa where it poses a major public health problem [2]. In Senegal, one person in ten, regardless of ethnicity, geographical origin or social class, carries the sickle cell disease gene [2].
Several complications can affect the development of sickle cell disease, including acute painful attacks, complications related to anemia, repeated infections and complications related to vascular thrombosis, including asplenia, which is one of the complications rarely encountered [1-8].
We report a case of asplenia in a 12-year-old boy with sickle S beta thalassemia.
B D is a 12 year old boy with known and followed sickle cell disease, a SAFA2 profile with 70.8% hemoglobin S, 19.7% Hb A; 8.6% Hb F and 0.9% Hb A2, compatible with an sickle S beta thalassemia profile. His history includes several hospitalizations for frequent painful crises, acute anemia and repeated infections. Three of his brothers have an undetermined (untested) profile and the fourth is a homozygous SS.
He was seen in consultation for severe abdominal pain. On clinical examination on admission, there was severe abdominal pain assessed at 9/10 on visual analogue scale (VAS), and anemia associated with severe jaundice. The rest of the exam was normal.
Laboratory results showed normocytic normochromic anemia (Hb: 8.5 g/dl; GMV=80.8 fL; HCMT=27.8 pg; hematocrit=24.1%). In addition, there was an increase in white blood cells at 19720 and platelets were 217,103/mm3.
The blood smear showed the presence of numerous acidophilic erythroblasts and Howells Jolly bodies.
On imaging, mesenteric lymphadenopathies were seen on ultrasound, the spleen was not visualized and there was also loss of liver and kidney gradient, associated with evidence of renal failure. Abdominal CT scan concluded that the spleen was defective (Figure 1).
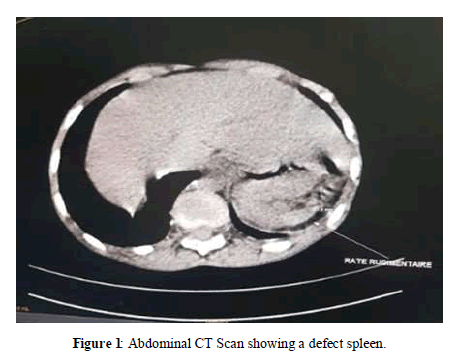
Figure 1: Abdominal CT Scan showing a defect spleen.
Patients with sickle cell disease are exposed to acute vaso-occlusive attacks and chronic visceral complications of ischemic origin that can affect virtually all organs. The spleen can also be the site of complete atrophy [3,4]. This occurs in homozygous sickle cell disease in childhood, around the age of about six years, after the congestion phase of the early years [3,4]. Repeated infarctions often lead to a progressive decrease in the size of the spleen (atrophy), which can lead to complete disappearance (asplenia). This complication remains rare. No cases have been reported in the Thiam [2] and Boiro [6] series in Senegal. However, in 20% to 30% of cases, the enlargement of spleen size may persist and even increase. Organ atrophy is also observed in the kidney of the sickle cell patient [4] Asplenia in our 12-year-old sickle beta thalassemia patient associated with signs of renal failure may suggest a functional form as reported by Owono, and N'Zi [3,4].
Asplenic people are generally prone to repeated, sometimes severe infections [3,5,8]. Our patient was hospitalized several times for infections related to his sickle cell. However, cases of lack of history of recurrent infections have been reported in the literature in asplenic patients [4,7].
Asplenia is a rare complication of sickle cell disease that must be considered in cases of repeated infections. Ultrasound and CT scan can help to effectively identify it.
Received: 13-Apr-2020 Published: 06-May-2020
Copyright: © 2020 Diouf JBN, et al. This is an open access paper distributed under the Creative Commons Attribution License. J ournal of Biology and Today's World is published by Lexis Publisher.