Mini Review - (2024) Volume 13, Issue 6
The KCNQ1/Kv7 channel, part of the Q1 subfamily of voltage-gated potassium channels, plays a key role in cardiac rhythm regulation and is commonly associated with Long QT syndrome (LQT1). Emerging evidence also suggests a role for KCNQ1 in insulin secretion, though its link to both hypersecretory and hyposecretory phenotypes complicates its connection between cardiac and metabolic syndromes. This complexity is further compounded by its epigenetic regulation and association with type 2 diabetes risk. The focal study investigated a unique case of Permanent Neonatal Diabetes Mellitus (PNDM) where KCNQ1/Kv7 dysfunction, typically linked to cardiac function, was identified as a novel contributor to the disease. Genetic analysis revealed a homozygous missense mutation in the KCNQ1 gene (C1189T/R397W) in a PNDM patient who did not exhibit overt cardiac symptoms. Using an in vitro Stem Cell (SC)-derived islet model and CRISPR/Cas9 gene editing, the study examined the mutation’s impact on pancreatic β-cell function. The results show that while the C1189T variant does not disrupt epigenetic regulation during pancreatic development or differentiation, it leads to a loss of KCNQ1 channel function, causing atypical electrophysiology. The SC model demonstrated that this impaired channel function results in a stage-dependent pattern of insulin secretion, characterized by initial hypersecretion followed by eventual pancreatic β-cell failure.
KCNQ1 • Permanent Neonatal Diabetes Mellitus (PNDM) • stem cell-derived islet modeling • insulin secretion • β-cell failure • KCNQ1 (C1189T/ R397W)
The KCNQ1 gene encodes the Kv7.1 potassium channel, essential for maintaining cardiac action potential and notably associated with Long QT syndrome (LQT1), a condition characterized by delayed cardiac repolarization and an increased risk of arrhythmias. Beyond its well- established role in the heart, KCNQ1 also plays a role in regulating insulin secretion from pancreatic β-cells [1]. However, its precise function in this context remains debated, as mutations have been linked to both hyperinsulinemia and impaired insulin secretion, underscoring a complex relationship between cardiac and metabolic syndromes [2-4]. Moreover, KCNQ1 is situated within an epigenetically regulated region linked to susceptibility to Type 2 Diabetes (T2D) [5-7].
The focal study addresses key gaps in the current understanding by investigating a rare case of Permanent Neonatal Diabetes Mellitus (PNDM), identifying dysfunction in the KCNQ1/Kv7.1 channel as a novel contributor to the disease [8]. The research focuses on a homozygous missense mutation in KCNQ1 (C1189T/R397W) discovered in a PNDM patient without obvious cardiac symptoms, revealing how this mutation affects pancreatic β-cell function. By leveraging an in vitro stem cell-derived islet model and CRISPR/Cas9 genome editing, the study provides important new insights into the KCNQ1 variant’s role in insulin secretion and β-cell viability (Figure 1).
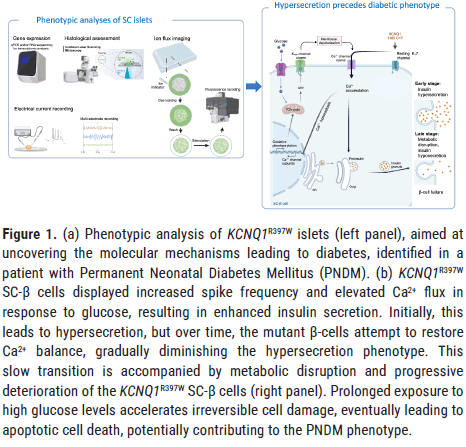
Figure 1. (a) Phenotypic analysis of KCNQ1R397W islets (left panel), aimed at uncovering the molecular mechanisms leading to diabetes, identified in a patient with Permanent Neonatal Diabetes Mellitus (PNDM). (b) KCNQ1R397W SC-β cells displayed increased spike frequency and elevated Ca2+ flux in response to glucose, resulting in enhanced insulin secretion. Initially, this leads to hypersecretion, but over time, the mutant β-cells attempt to restore Ca2+ balance, gradually diminishing the hypersecretion phenotype. This slow transition is accompanied by metabolic disruption and progressive deterioration of the KCNQ1R397W SC-β cells (right panel). Prolonged exposure to high glucose levels accelerates irreversible cell damage, eventually leading to apoptotic cell death, potentially contributing to the PNDM phenotype.
R397W mutation impairs KCNQ1 channel function
Although KCNQ1 is located in an epigenetically regulated region associated with type 2 diabetes, the C1189T mutation does not interfere with epigenetic processes during pancreatic differentiation [5-8]. Instead, this mutation alters the helical structure of the channel (Helix A), resulting in a loss of function in the Kv channel [8]. Notably, KCNQ1 channels are regulated not only by voltage but also by various interacting protein partners (e.g., calmodulin), modulatory co-factors (e.g., ATP), and ligands such as phosphatidylinositol 4,5-bisphosphate (PIP2) [9]. In particular, the R397W mutation has been shown to significantly impair the channel’s ATP sensitivity, suggesting that the R397 residue may contribute to forming an ATP binding site on KCNQ1 [10]. Rather than directly binding to the channel, ATP might modulate KCNQ1 function through interactions with regulatory proteins like calmodulin or by influencing the availability of different ligands, such as PIP2. Interestingly, PIP2 and ATP have been reported to compete in regulating the ATP-sensitive potassium channel (KATP) channel, where they act antagonistically: Mutations that enhance PIP2-mediated channel opening reduce ATP inhibition, leading to Neonatal Diabetes Mellitus (NDM). However, the potential interplay between ATP and PIP2 in regulating KCNQ1 function remains to be fully clarified [11,12].
SC-derived islet models for in vitro study
Diabetes research often relies on patient samples, cell lines and animal models, but these have limitations such as the scarcity of islets, variability in genetic backgrounds, and differences from human physiology. Human Stem Cell-derived (SC) islets offer a promising alternative, replicating the cell types and functions of human pancreatic islets. An unlimited supply of human islets from stem cells could significantly advance disease modeling and clinical applications. The main cell types in SC-islets consist of insulin-producing β cells, glucagon-producing α cells, and somatostatin-producing δ cells [13]. In vitro organoid models are especially valuable for examining the shift between hypersecretory and hyposecretory phenotypes, providing insights into conditions typically seen in late-stage patients [8,14]. Despite their similarities to primary pancreatic islets in terms of transcriptomes and function, SC-β cells exhibit different responses to insulin secretion triggers compared to primary human β cells. Additionally, they show reduced insulin secretion when transplanted into non-human primates, potentially due to dedifferentiation [15-17]. SC islets also face challenges like functional immaturity in vitro. Since β-cell secretory function develops only in the final differentiation stage, a maturation protocol was essential for studying the role of the KCNQ1/Kv7 channel in Glucose-Stimulated Insulin Secretion (GSIS). When this protocol was applied, the hESC_H1-derived KCNQ1R397W SC islet model showed effective responses to both GSIS and K+ Stimulated Insulin Secretion (KSIS) [8,17]. In contrast, patient-derived iPSCs could not be matured using the same approach. A deeper understanding of ion channel electrophysiology and function is still needed to improve the quality of in vitro cultured SC islets.
From hyperinsulinaemia to β-cell failure
The focal study demonstrated that electrophysiological signals from KCNQ1R397W SC-β cells exhibit an increased spike frequency in response to glucose stimulation, resulting in elevated Ca2+ flux during high glucose challenges [8]. This Ca2+ flux is essential for the formation of secretory insulin granules. These events enhance both Glucose-Stimulated Insulin Secretion (GSIS) and K+ Stimulated Insulin Secretion (KSIS) by promoting the exocytosis of insulin granules in β-cells. However, over time, the mutant β-cells attempt to restore Ca2+ homeostasis, leading to a gradual reduction in their hypersecretion phenotype. In the long term, these cells experience slow deterioration, with chronic high glucose exposure accelerating an irreversible process that ultimately results in apoptotic cell death, potentially contributing to the PNDM phenotype observed in the patient.
While previous interpretations have struggled to explain the conlicting reports of hypo and hyperinsulinemic phenotypes, a similar transition from hyperinsulinemia to insulin deficiency has been observed in other potassium channels. For example, dysfunction of the KCNH6 voltage-gated K+ channel results in a shift from hyperinsulinemia to hypoinsulinemia, leading to a diabetic phenotype [18]. Likewise, loss or reduced function of the ATP-sensitive KATP channel, observed in both KATP knockout mouse models and Congenital Hyperinsulinism (CHI) patients, mirrors the KCNQ1R397W phenotype, where an initial phase of insulin hypersecretion eventually progresses to a diabetic state [19]. Moreover, organoid studies with reduced KATP expression in MODY3 β-cells have shown that insulin hypersecretion precedes pancreatic β-cell failure, with a phase of initial hypersecretion followed by a transition to diabetes [14]. Collectively, these examples suggest that disruption of KCNQ1/KCNH6 or KATP channels can lead to short-term overstimulation of insulin secretion, eventually resulting in long-term β-cell failure.
Cardiovascular and metabolic links to KCNQ1
Although KCNQ1 is involved in both cardiac and pancreatic cell function, the link between the cardiovascular and metabolic disorders associated with KCNQ1 mutations is not always clear. The C1189T variant has been identified in an LQT1 patient, a condition associated with cardiac arrhythmias and sudden death, in a case of intrauterine death, and in a PNDM patient without cardiac symptoms [8,20,21]. The incomplete clinical penetrance in families with heterozygous KCNQ1 mutations partially explains the ambiguity in the manifestation of these conditions. Additionally, cardiovascular and metabolic syndromes may manifest at different life stages. For instance, LQT1 patients with dominant-negative KCNQ1 mutations have been observed to develop postprandial hyperinsulinemia, typically later in life [4]. On the other side, our PNDM patient is younger than the typical onset age for LQT1, so it may be too early to draw definitive conclusions about the cardiovascular aspects of his condition [8].
Distinct mechanisms of neonatal diabetes mellitus in KATP and Kv mutations
Although mutations in both ATP-sensitive (KATP) and voltage-gated (Kv) potassium channels have been associated with Neonatal Diabetes Mellitus (NDM), the mechanisms by which these mutations lead to NDM are fundamentally different. Activating or Gain-of-Function (GOF) mutations in KATP have been linked to human NDM, but the loss of β-cell mass in KATP-GOF mouse models is not due to apoptotic cell death. Instead, it results from a dedifferentiation process, where mature β-cells lose their identity and transition into insulin-negative cells [22]. In contrast, the mechanism in KCNQ1R397W mutations involves chronic overstimulation of β-cells, leading to their deterioration and eventual apoptotic cell death, which contributes to the NDM phenotype.
The study provides valuable insights into the complex phenotype associated with the KCNQ1R397W mutation. Although this mutation does not hinder pancreatic differentiation, it disrupts channel function, resulting in reduced insulin secretion, metabolic imbalance, and eventual apoptotic cell death. Despite experiencing intrauterine growth restriction and pancreatic cell loss, the PNDM patient with the KCNQ1R397W mutation survived [8]. However, the same mutation has been identified in an LQT1 patient linked to cardiac arrhythmias and sudden death, as well as in a case of intrauterine death, suggesting that the homozygous KCNQ1R397W mutation, potentially in combination with other factors, could be life- threatening. The conditions or factors influencing the severity or the transition between metabolic and cardiac phenotypes remain to be identified [20,21].
The study investigates the role of KCNQ1 in regulating insulin secretion from pancreatic β-cells. Through gene editing and the use of in vitro SC-islets, the research successfully elucidates the mechanisms behind both hypo and hypersecretion phenotypes. The in vitro models prove particularly valuable in their ability to replicate the shift in secretion phenotypes, suggesting that a hypersecretory phase may precede the onset of diabetes.
Although KCNQ1 mutations have been previously associated with cardiac disorders and type 2 diabetes, the study reveals a potential link between the KCNQ1 channel and a rare form of hereditary diabetes. The rarity of PNDM cases and the challenge of identifying patients with the same mutation present significant obstacles. Consequently, larger patient cohorts and additional research are needed to fully comprehend the impact of KCNQ1 mutations on hereditary diabetes, including PNDM.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Zhimin Z, et al. Atypical KCNQ1/Kv7 Channel Function in Neonatal Diabetes: Hypersecretion Prior to Pancreatic β-cell Failure. J Biol Todays World, 2024,13(6), 001-003
Received: 11-Sep-2024, Manuscript No. JBTW-24-147804; Editor assigned: 08-Sep-2024, Pre QC No. JBTW-24-147804 (PQ); Reviewed: 21-Oct-2024, QC No. JBTW-24-147804; Revised: 31-Oct-2024, Manuscript No. JBTW-24-147804 (R); Published: 11-Nov-2024, DOI: 10.35248/2322-3308-13.6.010
Copyright: © 2024 Zhimin Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.