Research - (2023) Volume 15, Issue 1
Introduction: Complications in COVID-19 are coupled With Acute Respiratory Distress Syndrome (ARDS) and Cytokine Release Syndrome (CRS). Interrupting IL-6 signaling by tocilizumab may be beneficial in those conditions.
Methods: We conducted retrospective observational study in patients with severe COVID-19 pneumonia receiving tocilizumab plus standard therapy. We compared respiratory support levels at hospitalization, before and after tocilizumab admission.
Results: Ninety-two patients fulfilled Serbian Therapy Protocol criteria and received tocilizumab in two separate doses (8 mg/kg i. v. per dose). Patients receiving conventional oxygen therapy before tocilizumab (max 15 l/min) showed significant decrease in respiratory support (z=-3.200, p=0.001) after the treatment, and lower mortality risk when compared to patients requiring high flow oxygen therapy before tocilizumab treatment (0.36 vs. 1.29).
Conclusion: Our results suggest beneficial effect of tocilizumab in addition to conventional therapy in respiratory support requirements, especially in earlier stages of ARDS and CRS.
Tocilizumab • COVID-19 • Respiratory support • Oxygen therapy
In December 2019, SARS-CoV2 changed the world we knew and triggered the greatest medical emergency in this century. WHO reports more than 600 million confirmed cases of COVID-19 and over 6 million deaths caused by multiorgan failure related to this virus. Clinical worsening and fatal outcomes are mostly induced by Acute Respiratory Distress Syndrome (ARDS), and cytokine release syndrome (CRS) [1, 2]. Both conditions are coupled with IL-6 signalization, leading to acute-phase proteins production and release, chemotaxis, increased vascular permeability, complement activation followed by intravascular coagulation, and further metabolic dysregulation [3, 4]. Considering this, it is likely to understand why IL-6 serum levels correlate to the level of respiratory failure and hypoxia, as some recent studies confirmed [5-7].
For this reason, WHO approved the use of anti-IL-6 receptor monoclonal antibodies (e.g. tocilizumab) for the treatment of severe COVID-19, since they are already accepted as the immunological therapy for rheumatoid arthritis, systemic juvenile idiopathic arthritis, giant cell arteritis, systemic sclerosis-associated interstitial lung disease, and CRS [8]. However, the available data about the efficiency in COVID-19 are controversial. While some authors report clinical recovery, lower mortality rate, and need for mechanical ventilation in groups treated with monoclonal antibodies for IL-6 receptor, the others find no benefits of this treatment [9, 10].
Serbian therapy protocol for COVID-19 recommends the use of tocilizumab in clearly defined indications, so we tried to examine whether tocilizumab in addition to standard therapy is capable of attenuating respiratory failure in those patients. We assumed that the use of tocilizumab will be beneficial, lowering the level of respiratory support and the need for mechanical ventilation.
We conducted an observational, retrospective study in patients suffering from severe COVID-19 pneumonia, hospitalized in specialized COVID hospital Krusevac, University Clinical Center Nis. The primary outcome was the change of respiratory support (lower as a positive outcome, and higher as a negative outcome). Secondary outcomes were the need for mechanical ventilation, discharge from hospital, or death. The data were collected from 1 January to 1 February 2021. We included only non-ICU patients receiving conventional oxygen support at simple face mask (max 15l/min)- conventional oxygen group –CO group, or High Flow Oxygen Therapy (HFOT) group-HF group, between 18 years and 90 years old, which fulfilled the criteria for tocilizumab treatment described by the 10th Serbian protocol for Covid-19 (positive nasopharyngeal swab for SARS-CoV2, moderate to a severe clinical condition, severe hypoxia requiring oxygenation, ARDS confirmed with RTG or CT, confirmed CRS with an increase of CRP>50 mcg/l coupled with IL-6>40 ng/l). Tocilizumab was administrated in two separate doses, 8 mg/kg i.v. (max. 800 mg) per dose to each patient.
Excluding criteria for our survey were: non-compliance for the criteria above, contraindications for tocilizumab, and patients already hospitalized in ICU.
Ninety-two consecutive patients in total fulfilled criteria and were analyzed. There were 56 (60.9%) men, and 36 (39.1%) women, aged between 37 years and 90 years old. Two more patients have met our criteria for follow-up but were intubated and admitted to ICU before the medicine admission, so were not considered in this paper.
All the patients also received standard therapy for COVID-19 defined by a current therapy protocol at the moment of hospitalization, which considered: oxygenation, antibiotics, vitamins, antiviral drugs (favipiravir), glucocorticoids (dexamethasone or methylprednisolone), and anticoagulants. Tocilizumab and glucocorticoids doses were calculated according to the body weight for each patient. Favipiravir was administrated only in patients with positive nasopharyngeal swab for SARS-CoV2, in less than five days from symptoms onset. Each patient fulfilling these criteria received Favipiravir twice a day for five days, 1600mg per dose for the first day, and 600mg per dose for the rest four days. Vaccines for COVID-19 were not available at that period of time in Serbia, so all the patients were not vaccinated.
Since the mortality caused by COVID-19 was enormous at that time, and Tocilizumab had shown significant beneficial effect, we decided to give the medicine to all the patients meeting criteria. Instead of having Tocilizumab and control group, we designed the study in the following manner: due to lack of a matched control group, we collected day-to-day data of respiratory support level for every patient, and set three-time intervals in order to extract data of respiratory support for each patient individually (T1hospital admission, T2-directly before tocilizumab administration, and T3 –up to 20 days after administration of the drug). These variables included levels of respiratory support at these three different time periods for each patient. For this purpose, we ranked the level of respiratory support from 0 to 4: 0-no oxygen support, 1-conventional oxygen at simple face mask (CO), 2-high flow oxygen therapy (HF), 3-non-invasive ventilation (NIVCPAP), and 4- Mechanical Ventilation (MV). Finally, we had three ordinary variables (OV): OV1 - respiratory support level at hospital admission, OV2respiratory support level before tocilizumab and OV3-respiratory support level after tocilizumab administration.
Respiratory support was determined according to following parameters:
• General condition,
• Arterial blood saturation
• Arterial blood gas analysis (PaOâ??/FiOâ??ratio) and
• Chest X-ray/MSCT.
Hemodynamics, saturation, and consciousness were monitored constantly, while samples of arterial blood were analyzed every morning, or more often if necessary. Patients underwent diagnostic imaging every 5 days-7 days, or in case of unexpected worsening. Respiratory support for non-intubated patients remained the same as long as saturation was above 90%, arterial pO2 above 50 mmHg, arterial pCO2 under 55 mmHg, consciousness preserved, and chest X-ray without signs of progression.
Escalation from conventional oxygen therapy to high flow oxygen therapy was done when a patient at simple facemask at 10-15L/min, did not achieve the set goal Spo2 above 90%. Criteria for escalation of oxygen therapy from HFOT to NIV or MV was persistent respiratory distressRR>40 breaths/ min, signs of labored breathing, use of accessory muscles of respiration, copious airway secretions, ABG: metabolic/respiratory acidosis, pH<7.25, PaO2 <55 mmHg, PaCO2 >55 mmHg, SpO2 <90% on current oxygen delivery device, signs of hemodynamic instabilityMAP<60 mmHg, requirement of inotropic support (norepinephrine>0.10 μgr.kg.min-1) with normal CVP, CRT>10 seconds, lactate ≥ 4.0 mmol/L, neurological impairment (GCS ≤ 8).
Weaning from HFOT may be commenced once the following criteria are met: patient has recovered from the severe acute respiratory distress syndrome (i.e., PaO2 /FiO2 >150), SpO2 >90% on FiO2 ≤ 0.4, no signs of respiratory distress such as agitation or anxiety, respiratory rate ≤ 25/min, heart rate ≤ 120/min, systolic blood pressure ≥ 90 mmHg, arterial pH ≥ 7.35.
Weaning completely from simple face mask oxygen therapy was done if clinical goal of Spo2-92% maintained after discontinuation.
Statistical analysis
Data were analyzed in IBM SPSS Statistics. In order to determine descriptive characteristics, we used Mean, Mode, Median, and Standard Deviation (SD). In order to determine the differences between groups, we used non-parametric tests of differences. For predicting treatment outcome we used discharge/death as dichotomous dependent variable for Binary Logistic Regression. In another Binary Logistic Regression, we used clinically improved/clinically got worse after tocilizumab administration as dichotomous dependent variable, while, for the same purpose, we used laboratory parameters (CRP, leukocytes, D-dimer, procalcitonin, AST, ALT, thrombocytes, ferritin, creatinine and IL-6), age, gender, time from symptoms onset to drug administration, and time from hospitalization until drug administration as independent variables.
We enrolled 92 patients in total, aged 37-90, (66.88 ± 11.75, 25% were younger than 59.5 years, and 25% were older than 74.75 years old). Laboratory parameters, gender and age distribution before and after tocilizumab treatment in CO and HF patients are shown in Table 1. Mean time from symptoms onset and hospital admission to tocilizumab administration were 8.7 ± 7.74, and 2.96 ± 5.14 days. Mean follow-up time was 10.67 ± 6.73 days per patient.
Table 1. Baseline laboratory parameters, gender and age distribution before and after tocilizumab treatment in CO and HF group.
| Before tocilizumab treatment | After tocilizumab treatment | ||||||
|---|---|---|---|---|---|---|---|
| Clinically improved* | Clinically got worse** | ||||||
| Group | Mean | SD | Mean | SD | Mean | SD | |
| CO | N | 60 | 48 (80%) | 12 (20%) | |||
| Gender: M/F | 36/24 | 34/14 | 2/10 | ||||
| Age (Years) | 66.68 | 11.981 | 66.42 | 12.079 | 67.75 | 12.039 | |
| CRP | 145.76 | 78.565 | 13.406 | 26.702 | 10.283 | 6.775 | |
| Leukocytes | 8.6 | 3.69 | 11.595 | 7.711 | 20.658 | 12.623 | |
| D-dimer | 2939.137 | 4579.354 | 1713.809 | 2866.458 | 6092.727 | 7110.617 | |
| Procalcitonin | 0.17 | 0.057 | 0.205 | 0.063 | 0.206 | 0.05 | |
| AST | 51.929 | 23.442 | 54.86 | 49.663 | 63.25 | 59.386 | |
| ALT | 51.754 | 34.101 | 120.488 | 100.653 | 77.166 | 53.046 | |
| Thrombocytes | 225.81 | 94.288 | 292.441 | 97.306 | 274.666 | 91.264 | |
| Ferritin | 1287.694 | 1296.363 | 618.435 | 486.783 | 903.383 | 970.258 | |
| Creatinine | 108.807 | 115.292 | 83.906 | 39.136 | 114.833 | 106.985 | |
| IL-6 | 87.546 | 73.162 | 136.35 | 309.516 | 442.333 | 410.543 | |
| HF | N | 32 | 15 (46.9%) | 17 (53.1%) | |||
| Gender: M/F | 20/12 | 12/3 | 8/9 | ||||
| Age (Years) | 67.25 | 11.481 | 60.8 | 10.234 | 70.29 | 11.947 | |
| CRP | 160.687 | 104.641 | 26.446 | 47.304 | 35.25 | 31.57 | |
| Leukocytes | 11.14 | 4.907 | 11.213 | 4.325 | 21.775 | 16.996 | |
| D-dimer | 3372.187 | 3882.782 | 2519.333 | 2326.82 | 5582.5 | 5007.135 | |
| Procalcitonin | 0.19 | 0.064 | 0.209 | 0.096 | 0.208 | 0.068 | |
| AST | 62.25 | 32.497 | 39.466 | 20.465 | 54 | 26.536 | |
| ALT | 83.531 | 89.361 | 116 | 131.528 | 79.666 | 79.538 | |
| Thrombocytes | 265 | 97.678 | 336.666 | 165.579 | 288.75 | 87.037 | |
| Ferritin | 1731.217 | 1376.983 | 1408.583 | 1447.448 | 957.562 | 686.891 | |
| Creatinine | 104.406 | 85.38 | 70.8 | 13.459 | 139.75 | 134.632 | |
| IL-6 | 85.203 | 74.139 | 685 | 304.055 | 606.125 | 406.786 | |
In order to determine the effect of tocilizumab on respiratory support modification, we compared mean ranks of respiratory support in threetime intervals mentioned earlier in the paper. A Friedman test indicated that there was a statistically significant increase in respiratory support from hospitalization (Md=2.13) to tocilizumab administration (Md=2.27) and a decrease after the treatment (Md=1.60), χ2 =31.098, p<0.001. Of sixty patients in CO group, after the treatment 80% of them did not require oxygen therapy, 1.7% remained on the same support level, and 18.3% had to be transferred to higher respiratory support (HF, NIV or MV) until the end of follow-up. A Wilcoxon Signed-Ranks test found a significant decrease in respiratory support level after the treatment (z=-3.20, p=0.001, effect size r=-0.41).
In the HF group counting thirty-two patients,43.8% of patients discontinued oxygen therapy, 3.1% continued oxygen therapy but on conventional O2 , 15.6% remained on HF, and 37.5% required stronger support (NIV and MV) after tocilizumab admission. Although the number of patients obtaining clinical improvement is not negligible, Wilcoxon Signed-Ranks testdid not find a statistically significant difference between respiratory support before and after tocilizumab (z=-0,79, p=0,43). Figures 1 and 2 show distribution of the patients according to respiratory support after tocilizumab administration in CO (Figure 1) and HF group (Figure 2).
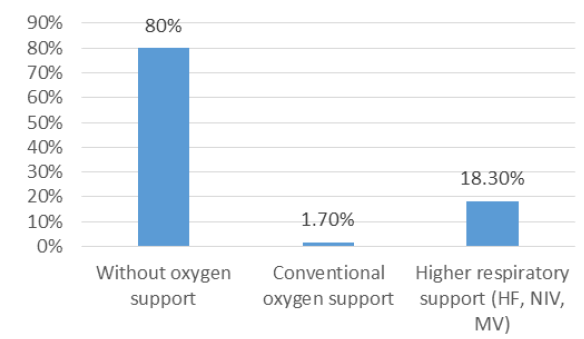
Figure 1: Distribution of patients according to respiratory. support after tocilizumab admission in the CO group.
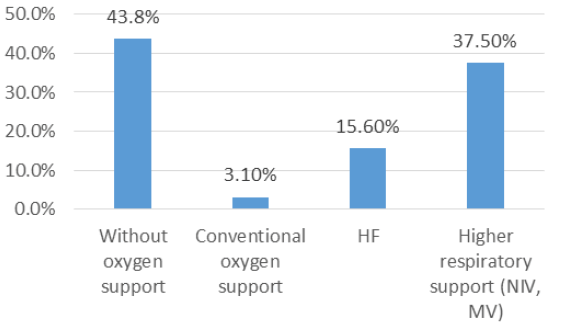
Figure 2: Distribution of patients according to respiratory support after tocilizumab admission in the HF group.
A logistic regression was calculated to predict treatment outcome (discharge/death) based on gender, age, time from symptoms onset to drug administration, time from hospitalization until drug administration, CRP, leukocytes count, D-dimer, procalcitonin, AST, ALT, thrombocytes, ferritin, creatinine, and IL-6. The logistic regression model was statistically significant, χ2 (14, N=92)=33.668, p=0.002. The model explained between 37.4% (Cox & Snell R2) and 50.7% (Nagelkerke R2) of the variance and correctly classified 83.3% of cases. As Table 2 shows, five independent variables were associated with treatment outcome (gender, time from symptoms onset to drug administration, time from hospitalization until drug administration, thrombocytes, and creatinine).
Table 2. Regression coefficients for predicting therapy outcome.
| Variable | B | S.E. | Wald | df | p | Exp(B) | 95% CI |
|---|---|---|---|---|---|---|---|
| Gender*** | 3.057 | 0.972 | 9.881 | 1 | 0.002** | 21.254 | [3.161, 142.925] |
| Age | -0.021 | 0.037 | 0.343 | 1 | 0.558 | 0.979 | [0.911, 1.051] |
| Time from symptoms onset to drug administration | 0.146 | 0.07 | 4.378 | 1 | 0.036* | 1.158 | [1.009, 0.315] |
| Time from hospitalization until drug administration | -0.579 | 0.295 | 3.854 | 1 | 0.050* | 0.561 | [0.315, 0.999] |
| CRP | 0.004 | 0.004 | 1.013 | 1 | 0.314 | 1.004 | [0.996, 1.012] |
| Leukocytes | 0.148 | 0.097 | 2.333 | 1 | 0.127 | 1.159 | [0.959, 1.402] |
| D-dimer | 0 | 0 | 0.065 | 1 | 0.798 | 1 | [1.000, 1.000] |
| Procalcitonin | 17.04 | 14.022 | 1.477 | 1 | 0.224 | 25139698.07 | [0.000, 2.166E+19] |
| AST | 0.021 | 0.017 | 1.491 | 1 | 0.222 | 1.021 | [0.988, 1.055] |
| ALT | -0.002 | 0.006 | 0.097 | 1 | 0.755 | 0.998 | [0.986, 1.010] |
| Thrombocytes | -0.018 | 0.008 | 4.337 | 1 | 0.037* | 0.983 | [0.966, 0.999] |
| Ferritin | 0 | 0 | 0.025 | 1 | 0.874 | 1 | [0.999, 1.001] |
| Creatinine | 0.008 | 0.004 | 4.382 | 1 | 0.036* | 1.008 | [1.001, 1.016] |
| IL-6 | 0.004 | 0.005 | 0.672 | 1 | 0.412 | 1.004 | [0.994, 1.015] |
Also, a logistic regression was calculated to predict discontinuation of oxygen therapy based on gender, age, time from symptoms onset to drug administration, time from hospitalization until drug administration, CRP, leukocytes, D-dimer, procalcitonin, AST, ALT, thrombocytes, ferritin, creatinine and IL-6. The logistic regression model was statistically significant, χ2 (14, N=92)=28.019, p=0.014. The model explained between 32.2% (Cox & Snell R2) and 44.5% (Nagelkerke R2) of the variance and correctly classified 81.9% of cases. As Table 3 shows three independent variables were associated with oxygen therapy discontinuation (gender, time from hospitalization until drug administration, and Thrombocytes).
Table 3. Regression coefficients for predicting discontinuation of oxygen therapy.
| Variable | B | S.E. | Wald | df | p | Exp(B) | 95% CI |
|---|---|---|---|---|---|---|---|
| Gender*** | 2.698 | 0.909 | 8.806 | 1 | 0.003** | 14.856 | [2.500, 88.292] |
| Age | -0.004 | 0.035 | 0.011 | 1 | 0.917 | 0.996 | [0.930, 1.068] |
| Time from symptoms onset to drug administration | 0.131 | 0.068 | 3.751 | 1 | 0.053 | 1.14 | [0.998, 1.302] |
| Time from hospitalization until drug administration | -0.575 | 0.284 | 4.093 | 1 | 0.043* | 0.563 | [0.323, 0.982] |
| CRP | 0.007 | 0.004 | 2.767 | 1 | 0.096 | 1.007 | [0.999, 1.015] |
| Leukocytes | 0.128 | 0.092 | 1.943 | 1 | 0.163 | 1.136 | [0.949, 1.360] |
| D-dimer | 0 | 0 | 0.052 | 1 | 0.819 | 1 | [1.000, 1.000] |
| Procalcitonin | 15.196 | 13.289 | 1.308 | 1 | 0.253 | 3978836.5 | [0.000, 8.154E+17] |
| AST | 0.029 | 0.017 | 2.87 | 1 | 0.09 | 1.029 | [0.995, 1.064] |
| ALT | -0.004 | 0.006 | 0.313 | 1 | 0.576 | 0.996 | [0.984, 1.009] |
| Thrombocytes | -0.017 | 0.008 | 4.439 | 1 | 0.035* | 0.983 | [0.967, 0.999] |
| Ferritin | 0 | 0 | 0.007 | 1 | 0.934 | 1 | [0.999, 1.001] |
| Creatinine | 0.002 | 0.004 | 0.203 | 1 | 0.652 | 1.002 | [0.993, 1.011] |
| IL-6 | -0.002 | 0.004 | 0.207 | 1 | 0.65 | 0.998 | [0.989, 1.007] |
Among all the patients, 19 were admitted to ICU and 14 underwent intubation. Fifty-eight patients were discharged from the hospital, from which 44 patients were receiving CO before tocilizumab administration, while the rest were receiving HF. Among the 34 patients who died, 16 were receiving CO before tocilizumab administration, while 18 were receiving HF. Patients receiving HF before tocilizumab admission had 3.54 times higher mortality risk when compared to patients receiving CO before the treatment (0.36 vs. 1.29) (Figure 3).
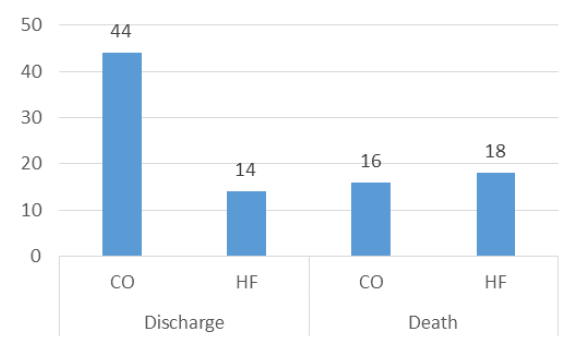
Figure 3: The treatment outcome for CO and HF group.
Mann-Whitney test did not find significant gender differences in respiratory support distribution at hospital admission (U=937, z=-0.708, p=0.479), or before tocilizumab (U=984, z=-0.233, p=0.816). However, the difference was obtained after the treatment (U=671.5, z=-3.242, p=0.001, effect size r=-0.338) revealing that women needed higher oxygen support than men (Md=55.85, n=36 vs. Md=40.49, n=56). While these gender differences were analyzed separately for three-time intervals (at hospital admission, before tocilizumab and after treatment), the Figure 4 combines these data into one image.
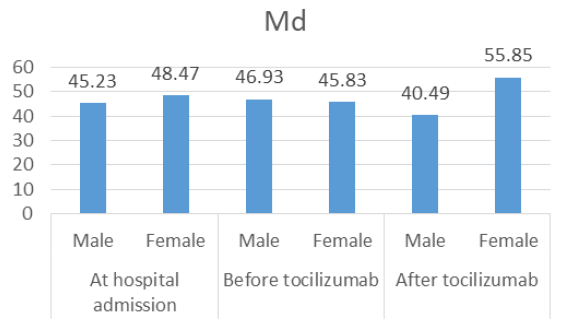
Figure 4: Respiratory support median distribution in male vs. female at hospital admission, before tocilizumab and after tocilizumabadministration.
SARS-CoV2 enters human cells by Angiotensin-Converting Enzyme (ACE) homolog-2 (ACE2) [11], the receptor that is highly expressed in the lungs [12]. Inflammation coupled with viral infection causes CRS mediated by IL-6 in up to 20% of cases of COVID-19, which is followed by more severe clinical conditions and increased mortality risk [13]. The reaction is also known as “cytokine storm” and is characterized by T-lymphocyte proliferation and enormous IL-6 release [14]. In interaction with IL-6 receptor, this cytokine stimulates acute-phase protein secretion, fever, leukocytosis, increased vascular permeability and hypotension, angiogenesis, intravascular coagulation, and multiple foci of inflammation [4,15-19]. The reaction in the lungs leads to tissue damage, alveolar fluid retention, and respiratory membrane thickening, ending with respiratory failure and hypoxia [20]. Considering pathogenesis, we hypothesized that interrupting IL-6 signalization pathway will significantly mitigate pulmonary damage, shown in the respiratory support demand.
Our results show that tocilizumab administration in group receiving CO support, as the lowest level of respiratory support, significantly improves hypoxia and reduces the need for further oxygenation, with gender and CRP as strongest predictors of discontinuation of oxygen therapy. On the contrary, in the HF group, we did not find a significant improvement. According to this, we concluded that in our patients tocilizumab treatment gave better results when applied in earlier stages of the disease, with less extensive lung damage. Also, we obtained clinical improvement, reduced ICU admission, need for MV, and mortality risk in patients with less severe pneumonia. We cannot exclude the importance of standard therapy, by which all the patients received glucocorticoids and anticoagulant therapy,and some of them antiviral therapy as well. We have also noticed that men in our group had better therapy responses, and a greater survival rate than women, supporting the idea of gender-based differences in the COVID-19 course and outcome.
Klopfenstein et al. received similar results in a retrospective casecontrol study at the very beginning of the pandemic [21]. They found tocilizumab administration in severe COVID-19 pneumonia to reduce the mortality and MV rate (27% in TCZ group vs. 52% in control group, p 0.009). Later, RECOVERY trial with 4116 patients in total and 2022 receiving tocilizumab, obtained the same (35% vs. 42%, risk ratio 0.84, 95%CI 0.770.92, p<0.0001) [22]. The meta-analysis by Snow et al. covering 10 RTC and 6 493 patients, of which 3358 treated with tocilizumab also evaluated the mortality risk and risk for MV [23]. They obtained significantly lower mortality risk in the tocilizumab group (24.4% vs. 29.0%; OR 0.87 (0.741.01); p=0.07; I2=10%; TSA adjusted CI 0.66-1.14), but not the risk for ICU admission, as well. Additional meta-regression confirmed the relation between tocilizumab and obtained results, especially in patients with more severe pneumonia.
One of our study limitations is the absence of the control group that would receive a placebo, but we designed the study as nest case control with evaluation of starting data and proportion of patients requiring higher or lower oxygen supply and we intended to compare data at the start and at the end of hospitalization. As we have mentioned earlier, this was the beginning of the second wave which threatened to overcome the previous one in mortality rate; Tocilizumab already showed some beneficial effect and was in the National Covid 19 guide, therefore we decided to give it to all the patients fulfilling criteria, hoping to lower complications and mortality. Further, our patients were mostly elderly people, with 25% older than 75 years old, and having one or more comorbidities which could have impacted on the disease course and therapy response. And finally, we had to use non-parametric tests for statistical analysis owing to distribution. Our study has some advantages, as well. All of the patients received the same standard therapy, following the protocol. We minimized the influence of other variables on the outcome by comparing the respiratory support before and after tocilizumab admission.
To conclude, our results are in concordance with other studies. The addition of tocilizumab to standard therapy in severe COVID-19 pneumonia seems to have a beneficial effect, especially when administrated in earlier stages of respiratory failure. More RTCs and meta-analyses are needed in order to form a definite opinion, but the results so far are encouraging.
Citation: Cvetanovic, V., et al. Influence of Tocilizumab on Respiratory Support Requirements in Non ICU COVID-19 Patients. Int. J. Collab. Res. Intern. Med. Public Health. 2022, 15 (1),1-5
Received: 21-Dec-2022, Manuscript No. IJCRIMPH-22-84352; Editor assigned: 23-Dec-2022, Pre QC No. IJCRIMPH-22-84352 (PQ); Reviewed: 05-Jan-2023, QC No. IJCRIMPH-22-84352 (Q); Revised: 08-Jan-2023, Manuscript No. IJCRIMPH-22-84352 (R); Published: 18-Jan-2023, DOI: 10.35248/ 1840-4529.23.15(1).1-5
Copyright: ©2023 Cvetanovic, V. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.