Research - (2022) Volume 14, Issue 5
Background: The Oxford-AstraZeneca COVID-19 vaccine was deployed in Nigeria on the 5th of March, 2021 with a goal of vaccinating 70% of the population by 2022. This goal is however being threatened by widespread vaccine hesitancy fueled by skepticism around vaccine safety. This study leverages on the clinical acumen of healthcare professionals to evaluate the occurrence of adverse events following immunization to the vaccine in tertiary hospitals in Nigeria.
Methods: It was a retrospective study conducted using a self-administered questionnaire from 25th May to 17th July 2021 in 4 tertiary hospitals across the north central zone of Nigeria. Participants were asked to report the type, intensity and duration of symptoms experienced following Oxford-AstraZeneca COVID-19 vaccine administration. Data was analyzed using SPSS version 22.0.
Results: Of the 295 vaccine recipients who took part in this study, around 71.1% of them reported experiencing at least one symptom with injection site pain (31.3%), fever (25.4%), tiredness (23.6%) and headache (22.9%) being the most commonly reported adverse events. Majority of the reported symptoms began within a day of vaccination; most had no impact on routine daily activities and almost two-thirds had resolved two days after their commencement. The frequency of reported symptoms was higher in: the younger (<40 years) age group, those who had taken two doses, and participants with a history of adverse reaction to a medicine/vaccine. These findings were consistent with those of clinical trials and similar studies in other regions.
Conclusion: As with other vaccines, some adverse events follow the administration of Oxford-AstraZeneca COVID-19 vaccine. Most of these events are short term, tolerable and similar to those reported in other parts of the world. Vaccine recipients should be informed about potential symptoms, how to handle them, and when and where to seek additional guidance if necessary. The findings in this study should help counter vaccine hesitancy by enhancing public confidence in Oxford-AstraZeneca COVID-19 vaccine safety.
Nigeria • COVID-19 • Oxford-AstraZeneca • ChAdOx1 nCoV-19 • Adverse effects • Vaccine
The widespread morbidity and mortality associated with the 2020 COVID-19 pandemic precipitated the most extensive and rapid global vaccine development program in history, culminating in several vaccine candidates attaining phase 3 efficacy milestones and receiving emergency use authorization by the end of the same year [1,2].
The AstraZeneca COVID-19 vaccine, codenamed ChAdOx1 nCoV-19, is a viral vector vaccine which uses the modified replication-deficient chimpanzee adenovirus, ChAdOx1 as a vector [3,4]. It was the first vaccine approved in Nigeria against COVID-19; it is stable at refrigerator temperatures making it more accessible to low- and middle-income countries with limited cold- chain capabilities [5]. It is administered as an intramuscular injection in a two-dose regimen with an interval of 12 weeks between doses [6]. Having demonstrated remarkable potency in clinical trials against the Wuhan strain [7-9] and new variants of SARS-CoV-2, the spotlight has been on the safety of ChAdOx1 nCoV-19. Nigerian Primary Healthcare Developmental Agency (NPHCDA) flagged off its nationwide deployment on the 5th of March 2021 to much fanfare and optimism. This optimism is however punctuated by lingering skepticism around vaccine safety due to widespread reports of thromboembolic events in some European countries and the conspiracy theories around the pandemic and vaccine. This negative spotlight has led to hesitancy in vaccine acceptance [10]. This survey leverages on the clinical acumen of Healthcare Professionals (HCPs) to facilitate formation of a national COVID-19 vaccine safety profile while also securing their buy-in to drive vaccine uptake by the general population.
The vaccine was shown to be safe and well tolerated in clinical trials [11]. Pain at injection site was the most commonly reported (58.5%) local Adverse Event Following Immunization (AEFI) during trials. The pain was mostly of mild intensity. Other local effects reported were redness, induration, and itching of site. Fatigue and headache were the most commonly reported systemic reactions. Other systemic effects were muscle aches, malaise, chills, and feeling feverish. This trend was similar across both the participants who took prophylactic paracetamol and those who didn’t, although the overall incidence was lower in those who took paracetamol. Most of these events began within the first few days of vaccination and no serious adverse effects were observed in the participants. This pattern of adverse reaction was similar across different age groups although the incidence decreased as the age increased. These findings were consistent with trials of other viral-vectored COVID-19 vaccines [12-24].
There’ve also been several post-licensure cohort studies to assess the safety of ChAdOx1 nCoV-19. Youssef et al. assessed safety of the first dose of vaccine among HCPs in Ethiopia [24] and found that 75.8% of vaccine recipients had symptoms like pain and tenderness at site, tiredness, headache, myalgia, back pain, fever, chills, diarrhea, vomiting, and runny nose. Few (6.1%) participants reported severe symptoms like dypsnoea, chest pain, leg swelling, abdominal pain, and persistent cough. None of the participants were hospitalized, most of the symptoms began within 24 hours of vaccination and majority had resolved by the third day. In a similar study, majority (94.6%) of participants reported at least one symptom after receiving ChAdOx1 nCoV19 [25]. The pattern of events was similar to that observed in Ethiopia [24]. Female gender and previous COVID-19 infection were associated with a higher incidence of symptoms, while chronic illnesses were associated with fewer symptoms. Some oral lesions including vesicles, blisters, ulcers, and bleeding gingiva were also reported from the study.
Studies from other regions also reveal a similar pattern of reported symptom only differing by occurrence of unique symptoms like sore throat, insomnia and impotence in Kabul, rash in Bangladesh, and a low incidence of fever in Republic of Korea [26-28].
Study design
It is a randomized, retrospective, multi-center, cross-sectional exploratory study carried out in four tertiary hospitals across 3 states of the north central geopolitical zone of Nigeria.
Inclusion/Exclusion criteria
All adults (18 years and above) HCPs who consented to being part of the study were included. Healthcare professionals who are less than 18 years of age as of 31st March 2021 were excluded.
Sample size
By NPHCDA figures for 31st March 2021, 54708 individuals had been vaccinated with ChAdOx1 nCoV-19 in the North Central zone. This was our population size. Using a confidence interval of 95%, a 6% margin for error, and allowing for 10% no-response rate, the minimum sample size was determined to be 296 by Cochran formular.
Sampling technique
A two staged simple random sampling technique was used to determine the states and then hospitals in which the study was conducted. Proportionate allocation of study subjects to each state was done based on the total number of people vaccinated as of 31st March 2021.
Study instrument
A self-administered, 45 questions questionnaire developed from review of literature was used [29-34]. The questionnaire was validated by six selected HCPs, prior to being pretested among fifteen randomly selected HCPs for average time taken to complete, legibility, clarity, and understanding.
Data collection
The study observed ethical standards of all relevant institutional committees and the Declaration of Helsinki. Prior to administering the questionnaires, each participant was given a consent form to complete following a detailed explanation of the study protocol.
Statistical analysis
The Statistical Package for the Social Sciences (SPSS) version 22.0 was used to carry out all statistical tests. Descriptive statistics were executed for the demographic data, medical anamnesis, knowledge of COVID-19 vaccine, and the reported adverse events of ChAdOx1 nCoV-19.
Ethical approval
This study was approved by the Health Research Ethics Committee of Bingham University Teaching Hospital, Jos, Plateau State. The approval number is NHREC/21/05/2005/00781.
295 HCPs participated in the study. There were slightly more male participants (50.8%) than females (49.2%). The mean age of the participants was 29.64 years with majority (61.7%) being in the 20-29 years age group (Table 1). Majority of the participants (96.9%) possessed tertiary level of education with over half being doctors (56.9%). Details regarding sociodemographics and medical anamnesis of the participants are provided in (Table 1).
Table 1: Demographic Characteristics of the Participants and Clinical History
| Variable | Frequency | Percentage (%) | |
|---|---|---|---|
| Gender | Male | 150 | 50.8 |
| Female | 145 | 49.2 | |
| Age group | 18-19 years | 4 | 1.4 |
| 20-29 years | 182 | 61.7 | |
| 30-39 years | 73 | 24.7 | |
| 40-49 years | 27 | 9.2 | |
| 50-59 years | 9 | 3.1 | |
| Level of Education | Primary | 4 | 1.4 |
| Secondary | 5 | 1.7 | |
| Tertiary | 286 | 96.9 | |
| Profession | Doctor | 168 | 56.9 |
| Nurse/Midwife | 44 | 14.9 | |
| Pharmacist | 33 | 11.2 | |
| Laboratory Scientist | 39 | 13.2 | |
| Other Allied Health Professional | 11 | 3.7 | |
| State of Residence | Abuja | 180 | 8.5 |
| Nassarawa | 25 | 61 | |
| Plateau | 90 | 30.5 | |
| Comorbidities | Asthma | 6 | 2 |
| Diabetes Mellitus (DM) | 9 | 3.1 | |
| Hypertension (HTN) | 15 | 5.1 | |
| HTN and DM | 4 | 1.4 | |
| Sickle Cell Disease | 2 | 0.6 | |
| None | 255 | 86.4 | |
| Previous Drug/Vaccine Reaction | Yes | 40 | 13.7 |
| No | 252 | 86.2 | |
| Food Allergies | Yes | 49 | 16.6 |
| No | 243 | 82.4 |
Despite majority of the participants (99.3%) reporting prior knowledge of ChAdOx1 nCoV-19, just over one-fifth of them (21.8 %) pinpointed the vaccine platform as viral vector (Figure 1). Most of the participants (98.0%) suggested there were adverse events associated with ChAdOx1 nCoV-19, and over two thirds (71.9%) that suggesting these events were common. Pain was postulated as the most common injection site symptom (81.4%), while headache (65.8%) and fever (65.8%) were the most common associated systemic symptom (figure 2).
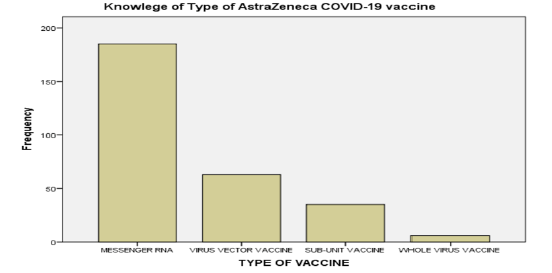
Figure 1: Knowledge of platform of the Oxford-AstraZeneca COVID-19 vaccine.
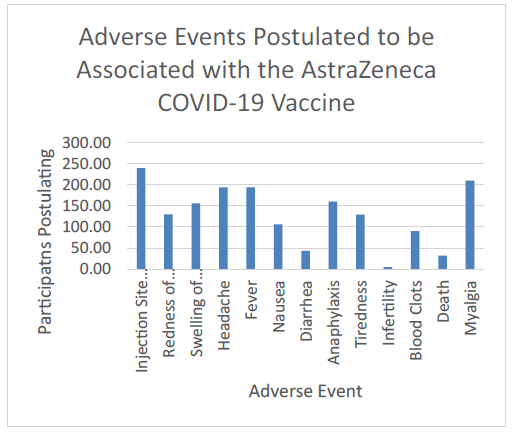
Figure 2: Postulated adverse events associated with the Oxford-AstraZeneca COVID-19 vaccine.
Most (96.3%) of the participants had received at least one dose of ChAdOx1 nCoV-19, with a higher proportion having completed the two doses (65.8%). Over two-thirds (71.1%) of vaccine recipients reported experiencing at least one AEFI. The incidence of AEFI was higher in those who had completed their two doses (77%) compared to the one dose recipients (59.8%). More males (73.1%) reported experiencing adverse events than females (69.1%).
As illustrated in (Figure 3), all vaccine recipients in the 50-59 years age group reported a symptom while among the younger age groups (<40 years) the highest incidence was seen in the 20-29 years group (70.7%).
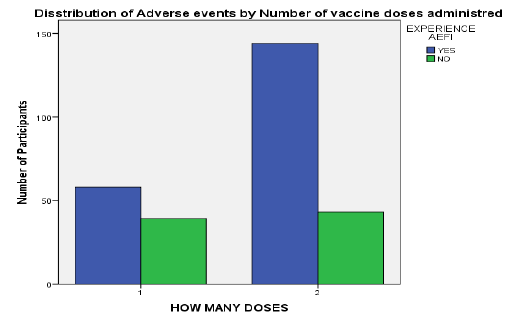
Figure 3: Distribution of reported adverse event by number of vaccine doses received.
A total of 391 AEFIs were reported by 202 (71.1%) vaccine recipients. Injection site pain (31.3%, 89) was the most commonly reported local AEFI (Figure 4 and Figure 5). Other local event reported was swelling of injection site (6%). Fever was experienced by about a quarter (25.4%) of vaccine recipients making it the most frequent systemic AEFI reported. Tiredness (23.6%) and headache (22.9%) followed closely. Other systemic AEFI reported were myalgia, nausea, and diarrhea. Chills and vomiting were reported by 2 participants each (Figure 5).
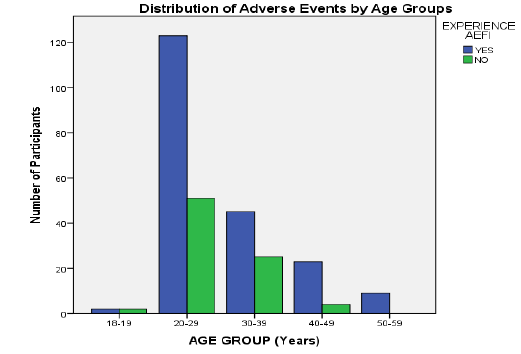
Figure 4: Distribution of adverse events reported by age groups.
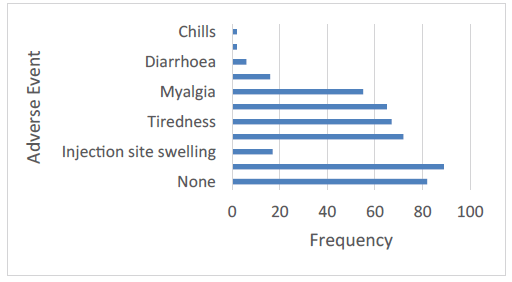
Figure 5: Reported adverse events following immunization with the AstraZeneca vaccine. Pain at the injection site was the most common local side effect, while feeling feverish or having a fever seemed to be the most common systemic side effects. 28.9% of participants reported having no side effects after their vaccination.
An overwhelming majority of the participants (96.3%) who reported having a food allergy also experienced an adverse event, while two-thirds of vaccine recipients who had experienced an adverse reaction to a medication or vaccine before reported an AEFI (65.8%). Most of the participants with chronic medical conditions also experienced an AEFI (72.2%). As shown in Table 2, all the reported AEFIs began within two days of vaccination with majority (68.3%) starting on the day of vaccination. Only one AEFI began two days after vaccination.
Table 2: Distribution of AEFI characteristics
| Variable | Frequency | Percentage (%) | |
|---|---|---|---|
| Onset of AEFI | The same day of vaccination | 269 | 68.3 |
| 1 day after vaccination | 124 | 31.5 | |
| 2 days after vaccination | 1 | 0.3 | |
| Severity of AEFI | Negligible | 201 | 51.2 |
| Unable to complete normal activity | 93 | 23.7 | |
| Unable to perform normal activity | 25 | 6.4 | |
| Took medications | 74 | 18.8 | |
| Resolution of AEFI | Same day AEFI began | 32 | 8.1 |
| A day after AEFI began | 134 | 34.1 | |
| 2 days after AEFI began | 116 | 29.5 | |
| 3 days to 1 week after AEFI began | 110 | 28 | |
| Second week | 1 | 0.2 | |
| Medications | Intravenous fluid | 8 | |
| Analgesic | 28 | ||
| Paracetamol | 45 |
Most of the reported AEFI were of mild to moderate severity with over half (51.2%) of them being negligible. Although some (18.8%) of the AEFIs required medical intervention mostly in the form of analgesics and antipyretics, they all improved within a few days (Table 2). Four of the participants required hospital admission for observation, they were discharged after a few days and recovered before the end of the week. An overwhelming majority (99.8%) of the AEFIs resolved within a week of their commencement with over two-thirds of them (71.7%) having resolved by the 3rd day. Only 1 AEFI extended into the second week (Table 2).
Despite being HCPs, the participants’ knowledge and awareness of ChAdOx1 nCoV-19 properties were inconsistent. While majority reported prior knowledge of the vaccine, just a handful accurately pinpointed the platform on which the vaccine was based. They however displayed commendable knowledge of the possible AEFIs with the vaccine though a handful associated the vaccine with infertility, blood clots, anaphylaxis and death, which was probably influenced by the sporadic reports of thromboembolic events in some European countries [7].
Majority of the participants reported experiencing at least one AEFI with pain at injection site, fever, tiredness, headache, and myalgia being the most commonly reported. Although the pattern of reported AEFIs was similar to that of the clinical trial of ChAdOx1 nCoV-19 [7], the frequency of these AEFIs was lower in our study. This could be due to differences in study sample demography, and psychological and race-based differences in symptom reporting behavior [35].
The distribution of reported AEFIs was also consistent with findings from other cohort post-licensure studies though there were some subtle differences observed. In the study among HCPs in Germany and Czech Republic [25], some oral lesions were reported as AEFIs while none of such were reported in this study, most likely because they were unsolicited for in this study.
Most of the reported AEFIs were of mild to moderate severity with over half reported as having no negative impact on routine daily activities. Less than one-fifth of symptoms required medications mainly in the form of analgesics and antipyretics, and they all improved in the following days. Since identifying and managing AEFIs vital to sustain trust in vaccines, WHO recommends taking analgesics for AEFIs [36]. However, CDC warns against taking analgesics prophylactically since the interaction between them and the vaccines are not yet known [36].
Four participants required hospitalization including a young laboratory scientist with a history of hypersensitivity to metronidazole. All hospitalized participants improved in the following days and they were all discharged in good health. No death was reported among the participants.
Over half of the reported symptoms had resolved within two days of their commencement and all except one was gone by the end of the first week. The onset and duration of reported AEFIs were consistent with those of other post-licensure studies [24-28].
AEFIs were reported more among those who had received two doses of ChAdOx1 nCoV-19 than those who had gotten just a single dose, which was consistent with findings from the vaccine’s clinical trials and a community-based study in Saudi Arabia.
Incidence of AEFIs was also higher in males than in females which was in contrast to observations from other cohort studies [25-27]. This might be attributed to the lower proportion of female participants in our study sample.
Although overall more AEFIs were reported by the younger participants (<40 years), the proportion of participants reporting AEFI was higher in the older age groups (>40 year). This was in sharp contrast with observations from both clinical trials and other studies [17-28]. This discrepancy could have resulted from differences in age distribution of sample populations (about 8.9% of participants in some clinical trials were aged 60 years and above whereas in this study the oldest age group [50-59 years] accounted for just 3.1% of participants) [16-17].
A higher incidence of AEFI was also seen in participants with a history of food allergy, and prior adverse reaction to a medication or vaccine. However, there wasn’t much difference observed in incidence of AEFIs among those with chronic illnesses, which contrasts with observations by Raid et al [25].
Although the small sample size of this study might make it difficult to generalize the findings, however, due to the lower coverage and relative shortage of vaccines in Nigeria, these findings could still shine some light on the situation and provide evidence against misunderstandings and conspiracy theories-backed beliefs driving vaccine hesitancy.
Also, the study instrument did not inquire about prior COVID-19 infection and its relationship with incidence of AEFIs. With several studies having shown a positive correlation between previous infection with SARS-CoV-2 and incidence of AEFIs, future studies should explore the role of prior infection and residual antibodies in the frequency and intensity of AEFIs.
As with other vaccines, some adverse events follow the administration of ChAdOx1 nCoV-19. The most common of these are injection site pain, headache, fever, tiredness and myalgia. Most of these events are short term, tolerable and similar to those reported by the manufacturing company. Within this study’s limits, the frequency of these events was higher in participants who - had completed the two doses of the vaccine, were males, had prior history of reaction to a drug/vaccine, and had known food allergies. Further independent studies are required to evaluate ChAdOx1 nCoV-19 overall safety especially in the long term, and also to estimate more precisely the incidence of each AEFIs.
Citation: Okezie KC. Adverse events following immunization with astrazeneca covid-19 vaccine among healthcare professionals in north central zone of Nigeria. Int. J. Collab. Res. Intern. Med. Public Health. 2022, 14 (05), 001-005
Received: 23-May-2022, Manuscript No. IJCRIMPH-22-64580; Editor assigned: 27-May-2022, Pre QC No. IJCRIMPH-22-64580(PQ); Reviewed: 30-May-2022, QC No. IJCRIMPH-22-64580(Q); Revised: 31-May-2022, Manuscript No. IJCRIMPH-22-64580(R); Published: 30-Jun-2022, DOI: 10.35248/1840-4529.22.14.3 41
Copyright: ©2022 Okezie KC. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.