Mini Review - (2024) Volume 16, Issue 1
Organoids are three-dimensional, self-organizing structures that mimic human organs and tissues, developed in vitro from stem cells or tissue samples. They offer advantages over traditional cancer cell lines and animal models by replicating the structure and function of human organs or tissues more accurately. Gastrointestinal organoids, which can be derived from the esophagus, stomach, intestine, liver, bile duct, or pancreas, have proven valuable in various applications. Additionally, organoids can mirror the cellular heterogeneity, morphology, and biochemical properties found in living organisms and can be customized to individual patients (known as Patient-Derived Organoids, PDOs). This article explores the tools and underlying mechanisms that make organoids a valuable asset in clinical research.
Organoids in drug screening and treatment response prediction: The use of Patient-Derived Organoids (PDOs) offers promising advancements in crafting personalized drug treatment plans, potentially enhancing patient outcomes. A study involving drug screening on 47 PDOs from biliary tract cancer patients tested seven commonly used chemotherapeutics and demonstrated varied therapeutic effectiveness depending on the individual patient’s material. PDO-based xenografts corroborated the screening results in 92.3% of cases. Additionally, an automated screening of 1172 FDA-approved drugs identified 26 that effectively targeted pancreatic cancer-derived PDOs, including 19 chemotherapeutics approved for other cancers [1-3]. Another study repurposed 34 anticancer drugs based on their effects on colorectal cancer PDOs, validated through in vivo PDObased xenografts. Transcriptome sequencing revealed five distinct drug response patterns [4].
A cutting-edge method for exploring cancer susceptibility genes involves using CRISPR/Cas9 screens with negative selection to identify genes whose knockout leads to specific phenotypes. In gastric cancer (GC)- PDOs, inhibition of histone lysine demethylase 1A (KDM1A) through genetic and pharmacologic means resulted in slowed organoid growth. KDM1A’s cancer-promoting role was linked to the suppression of N-Myc Downstream Regulated Gene 1 (NDRG1). Profiling of 20 GC-PDOs demonstrated that increased NDRG1 expression accurately predicts response to Lysine-Specific Histone Demethylase 1A (KDM1A) Inhibitors (KDMAi) with 100% sensitivity and 82% specificity [5].
These findings confirm that PDOs can predict the effectiveness of chemotherapeutic agents in precision oncology and facilitate drug repurposing. Advanced CRISPR/Cas9 technologies further enhance the potential of PDOs for exploring gene-drug interactions and discovering biomarkers.
Organoids in cancer modeling: Cancer modeling aims to create systems that replicate the development, progression, and behavior of cancer. Various methods use either Adult Stem Cells (aSCs) from normal tissue or Pluripotent Stem Cells (pSCs), such as Embryonic Stem Cells (eSCs) and Induced Pluripotent Cells (ipSCs). Applying CRISPR/Cas9 technology to aSCs allows for gene editing in organoids to study the effects of specific genetic alterations, such as driver mutations, on cancer development. Organoids generated using CRISPR/Cas9 with deficiencies in AT-Rich Interaction Domain 1A (ARID1A) have replicated the pathological features of cancer.
Despite their advantages, conventional CRISPR/Cas9 techniques in organoids have limitations, primarily due to the activation of the DNA damage response via the tumor-suppressor protein p53. This activation can cause large-scale deletions, insertions, or translocations. To address these issues, newer gene-editing methods such as base editing and prime editing offer promising alternatives by avoiding double-strand breaks. Base editing, utilizing either cytidine deaminase or adenosine deaminase, introduces specific point mutations (C–A or A–G) that may result in stop codons and protein truncation. Prime editing employs a Cas9 nickase combined with a Reverse Transcription (RT) domain and a prime editing guide RNA that includes the RT template, enabling precise insertions and deletions. Recent studies have demonstrated the effectiveness of base editing in tumor modeling with organoids. By using base editor multiplexing to target five cancer-related genes (APCQ1406, PIK3CAE545K, SMAD4R361H, TP53W53, and KRASG12) in a single step, researchers created a small biobank that accurately models colorectal cancer. This process involved two Cas9 variants, SpCas9-ABEmax and SpCas9- AncBE4max [6, 7].
Overall, the integration of advanced CRISPR/Cas9 technologies with organoid systems has greatly enhanced the development of tumor models, improving their fidelity and the accuracy of gene effect predictions.
Mucosoids for modeling infection with carcinogenic pathogens: In traditional 3D organoid structures, the apical surface is oriented towards the lumen, while the basal surface is exposed externally. This arrangement makes it challenging to study apical bacterial infections effectively. However, by converting 3D organoids into mucosoids and culturing them in a transwell chamber, researchers can better investigate infections caused by carcinogenic pathogens.
Mucosoids, when grown in an air-liquid interface culture, mimic in vivo conditions more closely. The basal side of these mucosoids interacts with soluble factors through the culture medium and produces mucus, similar to natural conditions. This setup is particularly useful for studying pathogens like Helicobacter pylori, a class I carcinogen that infects the gastric epithelium, and colibactin-producing Escherichia coli, which affects the intestine [8, 9].
The air-liquid interface allows for the introduction of additional cell types in co-culture systems. These can be placed either in the lower compartment of the trans well system or adhered to the trans well membrane. Furthermore, mucosoid cultures can be induced to undergo multiline age differentiation using a gradient of Epidermal Growth Factor (EGF) and Bone Marrow-Derived Protein (BMP), creating more realistic infection conditions for H. pylori. To study the effects of prolonged bacterial infections and their role in carcinogenesis, mucosoid cultures can be integrated into bioreactors or organ-on-a-chip systems that support growth in a perfused microenvironment. This integration, along with micro physiological systems, enables long-term co-cultivation of epithelial cells, stromal cells, and bacteria. Combining mucosoid cultures with these advanced systems represents a significant advancement in replicating the in vivo environment of bacterial infections (Figure 1) [10, 11].
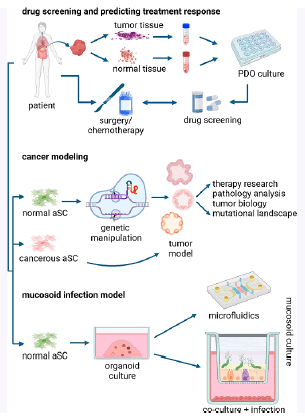
Figure 1: Uses of organoids in different experimental models.
This figure illustrates the diverse applications of organoids across various research models. It highlights how Patient-Derived Organoids (PDOs), sourced from tumor or adjacent normal tissues, are utilized for drug screening and predicting treatment responses, thereby impacting clinical decision-making and improving patient outcomes. The figure also showcases cancer modeling techniques that use Adult Stem Cells (aSCs) combined with advanced genetic manipulation methods to study tumor development and therapeutic strategies. Additionally, it demonstrates how transforming organoids into mucosoid cultures provides effective models for studying bacterial infections and intercellular communications over extended periods. This visual representation underscores the broad utility of organoids in both basic research and clinical applications.
Concluding remarks and future perspectives: Organoids and mucosoids have emerged as invaluable tools, greatly enhancing the scope of experimental cancer research. In both clinical medicine and experimental bioscience, Patient-Derived Organoids (PDOs) will become increasingly crucial for predicting treatment responses, thus supporting the advancement of precision medicine and personalized therapy regimens. The development of next-generation living biobanks and organoid-derived repositories will promote global scientific collaborations and expand our understanding of cancer.
Recent advances in gene editing, particularly with CRISPR/Cas9 technology, promise to transform our ability to assess genetic defects in tumors and develop tailored therapeutic approaches. Future research will also benefit from the evolution of more complex systems, such as assembloids. These 3D multicellular models and bioprinting techniques are expected to broaden the applications of these systems [12].
Mucosoids have become the new benchmark for infection modeling, providing a more accurate replication of gastrointestinal organs in vitro. To achieve long-term co-cultivation of epithelial cells, micro environmental cells, and bacteria, an integrated mucosoid culture with micro physiological systems will be essential. However, the application of mucosoids in cancer tissue studies remains limited due to the challenges cancer cells face in forming stable monolayers and maintaining cell-cell junctions.
Organoids and mucosoids have rapidly become essential tools in cancer research, significantly broadening the capabilities of experimental studies. These innovative models offer substantial advantages over traditional methods, particularly in the context of precision medicine and personalized therapies. Patient-Derived Organoids (PDOs), which are generated from individual tumor or adjacent normal tissues, are poised to play a pivotal role in predicting treatment responses. Their ability to closely replicate the unique characteristics of a patient's tumor allows for more accurate assessments of how different therapies might perform, thereby enhancing the efficacy of personalized treatment regimens.
The development of next-generation living biobanks and organoid-derived repositories is set to further revolutionize cancer research. By creating large, well-characterized collections of organoids, researchers can foster international collaborations and expand scientific knowledge on a global scale. These biobanks will enable comprehensive studies on tumor biology, treatment responses, and drug development, facilitating advances in both basic research and clinical applications.
Recent advancements in gene editing technologies, particularly CRISPR/Cas9, have the potential to transform cancer research by providing new tools to study genetic defects in tumors. These technologies allow for precise alterations in the genome, helping researchers understand the role of specific genetic mutations in cancer progression and response to treatment. As these gene-editing techniques continue to evolve, they will undoubtedly enhance our ability to develop targeted and effective therapeutic strategies tailored to individual genetic profiles.
Looking ahead, the emergence of more complex systems, such as assembloids, promises to expand the applications of organoid technology even further. Assembloids are sophisticated 3D multicellular models that integrate various cell types, offering a more comprehensive simulation of tissue and organ interactions. Combined with bio printing techniques, these systems can replicate intricate biological environments and processes, providing deeper insights into cancer development and treatment.
In summary, the integration of organoids and mucosoids into cancer research represents a significant leap forward in our ability to model disease, test treatments, and understand tumor biology. As these technologies continue to advance, they will undoubtedly drive further innovations in cancer research and therapy, ultimately improving patient outcomes and advancing the field of precision medicine.
The integration of organoids and mucosoids into cancer research marks a transformative advancement in the field, offering unparalleled insights into disease modeling, treatment efficacy, and tumor biology. These advanced models surpass traditional methods by providing more accurate and personalized approaches to cancer research and therapy. Patient-Derived Organoids (PDOs) play a crucial role in predicting individual treatment responses, thereby facilitating the development of tailored therapeutic strategies and enhancing the effectiveness of precision medicine.
The establishment of next-generation living biobanks and organoid-derived repositories represents a groundbreaking development, enabling global scientific collaborations and broadening our understanding of cancer. These resources are set to support extensive research on tumor biology and drug development, paving the way for significant advancements in both fundamental and applied cancer research.
Moreover, recent advancements in gene editing technologies, such as CRISPR/Cas9, offer powerful tools for investigating genetic mutations and their impact on cancer. As these techniques evolve, they promise to further refine our ability to develop targeted therapies and optimize treatment outcomes for individual patients.
Looking forward, the advent of more sophisticated systems, such as assembloids, will extend the capabilities of organoid technology. These 3D multicellular models, enhanced by bio-printing techniques, will provide more detailed simulations of tissue and organ interactions, leading to deeper insights into cancer mechanisms and therapeutic approaches.
In conclusion, the continued evolution of organoids and mucosoids holds the potential to revolutionize cancer research and treatment. By advancing our ability to model disease, predict responses, and explore new therapeutic avenues, these technologies will play a pivotal role in improving patient outcomes and advancing the future of precision medicine.
Citation: St. James, et al. Leveraging Gastrointestinal Organoids for Cancer TreatmentInt. Int J Collab Res Intern Med Public Health. 2024, 16, (1), 1-3
Received: 21-Jul-2024, Manuscript No. ijcrimph-24-143722; Editor assigned: 23-Jul-2024, Pre QC No. ijcrimph-24-143722; Reviewed: 01-Aug-2024, QC No. ijcrimph-24-143722(Q);; Revised: 06-Aug-2024, Manuscript No. ijcrimph-24-143722(Q);; Published: 15-Aug-2024
Copyright: ©2024 James St. this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.