Case Report - (2019) Volume 8, Issue 5
We are presenting a 2-year male child with large abdominal mass extending from right inguino-scrotal region to suprapubic region of size 4.0 × 5.0 cm. Mass causing pressure effect over bilateral kidneys and ureters with bilateral dilated renal pelvis and extending into the right inguinal region and pelvis. Histopathologically and immunocytochemically patient was confirmed as rhabdomyosarcoma of the retroperitoneum. Patient treated with six cycles of infusion chemotherapy with 3- weekly VAC regimen and was having progressive disease because of the aggressive behaviour of the disease and further treated with second line chemotherapy. The present case is a very unusual and rare site of metastatic presentation of the Rhabdomyosarcoma.<
Metastatic Rhabdomyosarcoma, RMS, Retroperitoneal mass, Giant Paediatric Tumor, VAC chemotherapy.
Sarcomas are a heterogeneous group of malignancies of mesenchymal cell origin that develop at primary sites throughout the body. Paediatric soft tissue sarcomas form a heterogeneous group of non-epithelial extra skeletal malignancies, representing 7% of all childhood tumors, approximately half of which are Rhabdomyosarcomas and the rest are Nonrhabdomyosarcomatous soft tissue sarcomas.
Rhabdomyosarcoma (RMS) is the commonest, highly malignant soft tissue sarcoma of childhood and adolescents, which accounts for 3% of all pediatric tumors [1]. Incidence peaks in children aged 1-4 years [2]. Tumors located in the trunk, the upper and lower limbs occur more frequently in adolescents and are generally the alveolar type. Only around 13% of RMS has evi-dence of metastatic disease at the time of the initial presentation [3]. Here, we are presenting a very unusual case of metastatic RMS.
A 2-year-old male child presented with history of lump in right inguino-scrotal region and abdominal mass since two months. Scrotal lump gradually increased in size and was associated with abdominal guarding and tenderness. There was no history of associated pain, fever or any significant past or medical history. Systemic examination revealed hard indurated abdominal mass extending from right inguino-scrotal region to suprapubic region of size 4.0 × 5.0 cm.
Complete hemogram and routine blood biochemistry parameters of the patient were within normal limits.
Chest radiograph of the patient was normal. Contrast enhanced computed tomography (CECT) scan of abdomen revealed a heterogeneously enhancing mass lesion measuring 3.7 × 3.0 × 4.7 cm in right side of scrotum. Another large heterogeneously enhancing mass lesion was seen in retro peritoneum extending up to pelvis. The mass was displacing gut loops and showing pressure effect on bilateral kidneys (Figure 1).
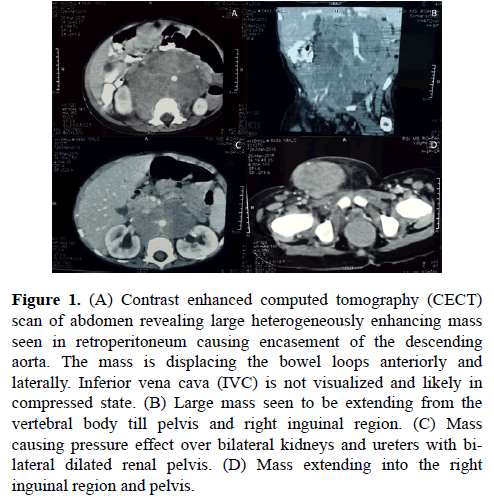
Figure 1. (A) Contrast enhanced computed tomography (CECT) scan of abdomen revealing large heterogeneously enhancing mass seen in retroperitoneum causing encasement of the descending aorta. The mass is displacing the bowel loops anteriorly and laterally. Inferior vena cava (IVC) is not visualized and likely in compressed state. (B) Large mass seen to be extending from the vertebral body till pelvis and right inguinal region. (C) Mass causing pressure effect over bilateral kidneys and ureters with bilateral dilated renal pelvis. (D) Mass extending into the right inguinal region and pelvis.
Cytopathology of the retroperitoneal mass was having dilemma between rhabdomyosarcoma and malignant small round cell tumor. Patient underwent retroperitoneal lymph node dissection with orchiectomy of the affected side. The neoplastic cells illustrated wide spread positivity for Myogenin, Desmin and B-cell lymphoma 2 (BCl2) stain and stain for Cytokeratin (CK), Leukocyte Common Antigen (LCA), Smooth Muscle Actin (SMA), S-100, PLAP, CD-30, CD-56, CD-99, CD-117, Vimentin and Synaptophysin were negative (Figure 2). Histopathologically and immunocytochemically patient was confirmed as rhabdomyosarcoma of the retroperitoneum. With this diagnosis, patient was treated with six cycles of infusion chemotherapy with 3-weekly VAC regimen (vincristine, doxorubicin and cyclophosphamide). After completion of 6-cycles of VAC chemotherapy, patient was presenting with progressive disease and further decided to give six more cycles of three weekly, second line infusion chemotherapy with carboplatin plus etoposide (carboplatin day 1 and etoposide day 1 to 3). Presently, patient is having non-progressive disease and on regular follow-up.
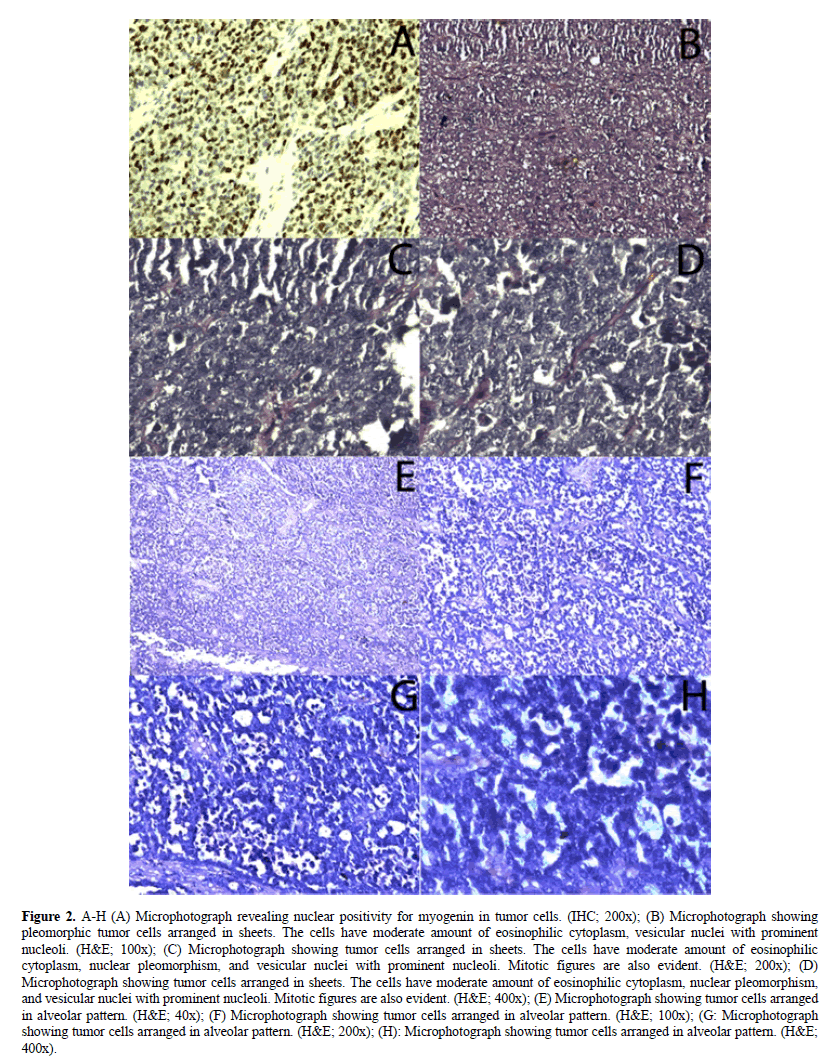
Figure 2. A-H (A) Microphotograph revealing nuclear positivity for myogenin in tumor cells. (IHC; 200x); (B) Microphotograph showing pleomorphic tumor cells arranged in sheets. The cells have moderate amount of eosinophilic cytoplasm, vesicular nuclei with prominent nucleoli. (H&E; 100x); (C) Microphotograph showing tumor cells arranged in sheets. The cells have moderate amount of eosinophilic cytoplasm, nuclear pleomorphism, and vesicular nuclei with prominent nucleoli. Mitotic figures are also evident. (H&E; 200x); (D) Microphotograph showing tumor cells arranged in sheets. The cells have moderate amount of eosinophilic cytoplasm, nuclear pleomorphism, and vesicular nuclei with prominent nucleoli. Mitotic figures are also evident. (H&E; 400x); (E) Microphotograph showing tumor cells arranged in alveolar pattern. (H&E; 40x); (F) Microphotograph showing tumor cells arranged in alveolar pattern. (H&E; 100x); (G: Microphotograph showing tumor cells arranged in alveolar pattern. (H&E; 200x); (H): Microphotograph showing tumor cells arranged in alveolar pattern. (H&E; 400x).
Rhabdomyosarcoma (RMS) is the commonest, highly malignant soft tissue sarcoma of childhood and adolescents, which accounts for 3% of all pediatric tumors [4]. Incidence peaks in children aged 1–4 years, lower in children aged 10–14 years and lowest between 15-19 years [5]. RMS is derived from immature striated skeletal muscle; hence, this disease can virtually occur anywhere in the body, though there are distinct clinical patterns according to the age at presentation, the histologic subtype and the site of the tumor. Head and neck tumors, including those in parameningeal locations tend to occur in children less than 8-years of age and are usually the embryonal type. Tumors located in the trunk, the arms, or the legs occur more commonly in adolescents and are usually the alveolar type. Bladder and vaginal tumors tend to occur in infants and in very young children and are the botryoid type of embryonal rhabdomyosarcoma. Only about 13% of patients with RMS have evidence of metastatic disease at the time of the diagnosis. The lung parenchyma is the most common site of metastasis, followed by bone marrow, bone and locoregional lymph nodes [6]. The present case is a very unusual and rare site of metastatic presentation of the RMS.
Patients with primary metastatic or recurrent RMS have a very poor prognosis, but the prognosis of patients with localized RMS has improved significantly with multidisciplinary management in the last two decades with an event-free survival (EFS) rate of approximately 70% [2]. Primary disseminated tumors in the Intergroup Rhabdomyo-sarcoma Studies IRS I, II and III have shown 5-year survival rates of patients between 20-30% [1,7-9].
Current therapy of RMS involves use of several treatment modalities like surgery, radiotherapy and chemotherapy. The cure rate with localized RMS has markedly improved three times over the past 2-decades, but children’s with metastatic disease at presentation have not much benefited and urgently need innovative therapies. Patients with metastatic RMS, with age more than 10-years and with embryonal RMS, have estimated long-term EFS of less than 20% [1,7-9].
Staging procedures include computed tomography (CT) or magnetic resonant imaging (MRI) studies of the primary site, CT scan of the chest, bone scan, bone marrow aspirates/biopsies, and lumbar puncture for the parameningeal localized sites. Several imperative prognosticators have been found in recent treatment strategies and patients are categorized accordingly to guide risk adapted therapy. Risk factors include the site of the primary tumor; the magnitude of the initial surgical resection; the age at diagnosis, with infants and adolescents generally faring less well than children 2-10 years old; the histologic type; the tumor–node– metastasis (TNM) stage and response to therapy.
The treatment approaches includes surgical resection, chemotherapy, radiation therapy individually or in combination. Chemotherapy remains the mainstay of treatment to reduce the size of the primary tumor and to eradicate gross or micro-metastases. Complete resection cannot be achievable in most of the patients because of the location of majority of RMS's. Radiotherapy is mostly used to control residual bulky or microscopic disease, particularly when the tumor is located in nonfeasible sites for surgery [2].
In the last three decades, there is a revolution in the treatment strategies for RMS of the head and neck region. Long-term comparative studies and metaanalysis helped the international medical fraternity to create new treatment modalities that are composed of combination chemotherapy, radiation therapy and surgery and this has significantly improved the prognosis in majority of the patients. According to American and European statistics data, the overall 5- year survival rate is now 73% and 71% re-spectively [9]. Unfortunately, outcome of the patients with metastases at the time of di-agnosis are still suspicious and these accounts for 15% of all pediatric patients with RMS and their prognosis has not much improved in last 15-years with a 5-year survival rate of only 20–30% [2,7-9]. The disease is challenging with recurrent tumor though temporal complete remission after second line treatment is possible but with poor probabili-ties for complete recovery [10].
The most effective chemotherapy agents against rhabdomyosarcoma cells are vincristine, doxorubicin, cyclophosphamide, dactinomycin, ifosfamide and etoposide. Polish Pediatric Solid Tumors Group usually recommends CWS therapeutic protocols for the management of RMS. For group IV patients i.e., with disseminated tumor dis-ease, aggressive chemotherapy is usually recommended, subsequent with autologous myogenic stem cell transplantation but is still having worst prognosis [11].
The Radiation therapy is used in most of the RMS cases and its dose and fractionation schedule are individualized according to the therapeutic protocols. Surgical treatment and radiation therapy are reserved for patients of first-line treatment failure. According to American Therapeutic Protocols, surgery and radiation therapy are considered at earlier stages of treatment. Radiation dosage usually preferred is between 36-50.4 Gy, however, smaller dose can be used in group II patients with incomplete surgery. Patients with residual disease or with unresected tumor require higher radiation doses. The real challenge for radiation oncologists are the children under the age of 3-year, in whom the risk rate is significantly high. Because of many critical structures in the head and neck region, late effects of irradiation are frequent. In order to minimize the risk of radiation i.e., to increase the safety of radiation therapy, now a days conformal radiotherapy, intensity-modulated radiation therapy (IMRT), image guided radiation therapy (IGRT) or proton therapy are usually preferred [12]. Brachytherapy, now a days has also emerged with promising results, because of the fact that radiation dose given directly to the tumor bed are having lesser complications as compared to conventional radiotherapy and has shown good results particularly in RMS of the genitourinary tract and extremities.
Surgical management of RMS is an important component of the multifarious management strategy as complete resection and has the best prognosis. However, due to the complex anatomical structure of the head and neck region, it is extremely difficult to achieve margin free tumor resection. In addition, the RMS of the head and neck is already locally advanced in more than 50% of patients at the time of presentation [13]. Therefore, if the surgical resection is incomplete or the risk of disfigurement and loss of function is high, induction chemotherapy can be the choicest step and surgical intervention is limited for the diagnostic biopsy only [11]. Histopathological examinations of all the clinically suspected lymph nodes are strongly recommended. Patients who are excluded from the primary tumor resection may undergo second-look surgery after receiving neoadjuvant chemotherapy or chemoradiation. The most common surgical complication includes paralysis of trigeminal and facial nerves, compromised motility of the temporo-mandibular joint and cosmetic defects.
In terms of conventional chemotherapy, escalated chemotherapy regimen for non-metastatic RMS provided no survival advantage but adds toxicity when the ifosfamide, vincristine, and actinomycin D (IVA) schedule was compared with the six-drug combination (IVA plus carboplatin, epirubicin, and etoposide) [3]. At the same time, intravenous vinorelbine 25 mg/m2 on days 1, 8 and 15 of each 28-day cycle along with continuous low doses oral cyclophosphamide 25 mg/m2 showed fascinating re-sponse rate in RMS [14]. Further concurrent radiotherapy (in the range of 30.6–50.4 Gy) with irinotecan and carboplatin in RMS demonstrated favourable tolerability, efficacy, and local control [15]. Based on the immunology and genetic mapping, newer therapeutic approaches may provide important prognostic information and can be future revolution in the management of RMS [6].
The present case of 2-year male child is a very unusual and rare site of metastatic presentation of the RMS. CECT abdomen which is responsible for staging and extent of the disease, revealed a large heterogeneous mass in the retroperitoneum extending up to pelvis. Histopathology with immunocytochemistry finally established the diagnosis of RMS. Only about 13% of patients with RMS have evidence of metastatic disease at the time of the diagnosis. Patients with primary metastatic or recurrent RMS are still more pessimistic and have poor prognosis with EFS of 15%. Combination chemotherapy like VAC is the mainstay of treatment because complete resection is usually not feasible in most of the patients and radiation therapy is mostly used to control residual bulky or microscopic disease. The genetics of the tumor cells is the future for the disease which may provide important prognostic information. Finally, the authors conclude that retroperitoneal RMSs are extremely rare tumors with poor prognosis and only infinitesimal cases have been reported in the literature, hence the conclusions about treatment and prognosis are equivocal. However, the best approach for treating these malignant tumors is the collaboration between the paediatric surgeon, the pathologist, and the oncologists in order to optimize the better treatment outcomes for the best interest of the patient.
Received: 23-Nov-2018
Copyright: © 2019 Anil Kumar Dhull et al. This is an open access paper distributed under the Creative Commons Attribution License. Journal of Biology and Today's World is published by Lexis Publisher; Journal p-ISSN 2476-5376; Journal e-ISSN 2322-3308.