Case Report - (2022) Volume 3, Issue 5
In the field of molecular modeling, Molecular Docking is the computational modeling of the structure of complexes formed by two or more interacting molecules. The goal of molecular docking is the prediction of the three-dimensional structures of interest. Molecular docking has become a progressively important tool for drug discovery. Molecular docking and molecular dynamics simulations are widely used in combination to predict the binding modes and stability of different protein-ligand systems. Protein-ligand interaction is an imperative subject in structure-based drug design and protein function prediction process. With advancements in computational power, molecular dynamics simulation is now a fundamental tool for investigative bio-molecular assemblies at the atomic level. In this review, I have focused on protein-ligand interactions using molecular docking, virtual screening, and molecular dynamics simulations. Here, I covered an overview of the available methods for molecular docking and molecular dynamics simulations, and their advancement and applications in the area of modern drug discovery, in medicinal plants. Molecular docking and molecular dynamics simulations are widely used in combination to predict the binding modes and stability of different protein-ligand systems. Protein-ligand interaction is an imperative subject in structure-based drug design and protein function prediction process. With advancements in computational power, molecular dynamics simulation is now a fundamental tool for investigative bio-molecular assemblies at the atomic level.
Protein-ligand interaction • Structure-function relationships • Docking • Molecular dynamics simulation • Drug discovery
Signal transduction relies heavily on the interactions of biologically important components such as proteins, nucleic acids, carbohydrates, and lipids. Proteins are fundamental components of living creatures that are involved in a wide range of biological processes. To accomplish their tasks, proteins normally bind to other molecules with high specificity and affinity. The shape of their binding pocket and the physicochemical properties of the amino acid residues creating the binding cavity determine how well proteins attach to other molecules. The interactions between protein and ligands are essential for many biological functions. The intermolecular interactions between protein and ligand can be electrostatic, van der Waals, hydrophobic interactions, etc. Molecular Docking is a method that anticipates the favored orientation of ligand against a receptor (Protein) to make a stable complex.
Most protein-ligand docking attempts aim to predict and categorize probable molecular complexes based on the three-dimensional structure of the ligand-binding site, binding poses, and binding energetics, showing the molecular recognition pattern between proteins and ligands. To estimate the affinity and activity of small molecules, the molecular docking technique is widely used to predict the binding orientation of drug candidates to their protein targets. When two molecules are coupled together to form a stable complex, docking is a method to predict one molecule's preferred orientation to the other. Scoring functions are used to determine the strength of an association or binding affinity between two molecules based on the preferential orientation of the molecules[1].
Molecular recognition is a biological process that develops particular connections between two or more bio-molecules to establish a range of biological processes such as central dogma, cell signaling, transportation, and regulation, enzyme catalysis, pathogenic infections, and immune reactions. The docking technique consists of two main steps: identifying the binding affinity and modeling the ligand structure, as well as its position and orientation within these sites (called pose). These two steps deal with sampling procedures and scoring schemes, both of which will be discussed in the theoretical section. A pose is generated, scored, and compared to the previous pose during computational docking. Based on the score for that instance, the current pose is then accepted or refused. After that, a new pose is formed, and the search process continues until it reaches an endpoint. In docking, searching and scoring can thus be strongly connected. Natural goods have been a rich source of medicinally active ingredients for millennia, and they have played a critical role in the treatment and prevention of an endless number of ailments. Natural items contain a vast amount of historic knowledge. When it was successfully combined with traditional medicine in the management of the SARS outbreak, the importance of this incoming pandemics was demonstrated. As a result of their important role in the SARS-CoV outbreak.
Medicinal plants are a rich source of structurally unique chemicals that can be used as a starting point for developing new medications. Herbal medicine has the advantages of being non-injectable, viable at room temperature, and having no significant adverse effects. To prevent immune system impairment, which is required for recovery from SARSCoV- 2 infection, the herbal mixture can also be used as a health drink, health food, or nutritional supplement.
There are 2 types of docking.
• Rigid docking
• Flexible docking
We're seeking a transformation in the 3D space of one of the molecules that make it an optimal fit with the other molecules in terms of a scoring function if the molecules are stiff. The ligand can be confirmed in the absence of receptor binding activity or the presence of receptor binding activity.
Our goal is to uncover the confirmations of the receptor and ligand molecules, thus we evaluate molecular flexibility in addition to transformation.
Molecular docking approaches
The perception of molecular complementarity, in which two structures interact like a hand in a glove, is the basis for molecular docking. The primary premise for estimating the binding and fitting of two molecule structures is form complementarity. Within the molecular docking community, two techniques are particularly prominent. A corresponding approach in which the proteins and ligands are described as complementary surfaces is used in one way. The second method duplicates the docking procedure by estimating the ligand-protein pairwise interaction energies, which is accomplished using the following methods[2].
Monte Carlo Approach: It establishes the initial configuration of a ligand in an active site, including random conformation, translation, and rotation. The first setting is given a score. It then generates a new arrangement and assigns a score to it.
Fragment-based method: Division of the ligand into separate protons or fragments, docking of the fragments, and finally linking of the fragments are all examples of fragment-based methods.
Distance Geometry: Intramolecular or intermolecular distances can be used to represent a variety of structural data. The distance geometry concept makes it possible to put these distances together and calculate three-dimensional structures that are consistent with them.
Matching approach: The emphasis of these strategies is on complementarity. The ligand atom is positioned in the "optimal" position for the site, resulting in a ligand-receptor configuration that may require modification.
Ligand fit approach: The phrase "ligand fit" describes a fast and accurate method for docking small molecule ligands into protein active sites while taking form complementarity into account.
Point Complementarity approach: These methods are based on evaluating a shape or chemical complementarity.
Blind Docking: It was developed to detect potential peptide ligand binding sites and modes by scanning the full surface of target proteins.
Inverse Docking: A computer technology was used to determine the toxicity and side effects of protein targets of a small chemical in this study. The combination of these targets and the proteome pharmacokinetic profile can aid in the evaluation of potential toxicities and side effects of a drug candidate.
• DOCK (1982,2001)
• FleX (1996)
• Hammerhead (1996)
• Surflex (2003)
• SLIDE (2002)
• AutoDock (1990,1998)
• ICM (1994)
• MCDock (1999)
• GOLD (1997)
Basic requirements for molecular docking
A ligand docking method requires a target protein structure, the molecules of interest, or a database of real or virtual compounds for the docking process, and a computational framework that allows the needed docking and scoring processes to be implemented. Many docking approaches assume that the protein is stiff; nevertheless, the binding pose in the protein's binding pocket must be considered when it comes to the conformational degree of freedom. To attach stiff molecules or fragments to protein active sites, several approaches such as clique-search, geometric hashing, and pose clustering can be applied (Figure 1).
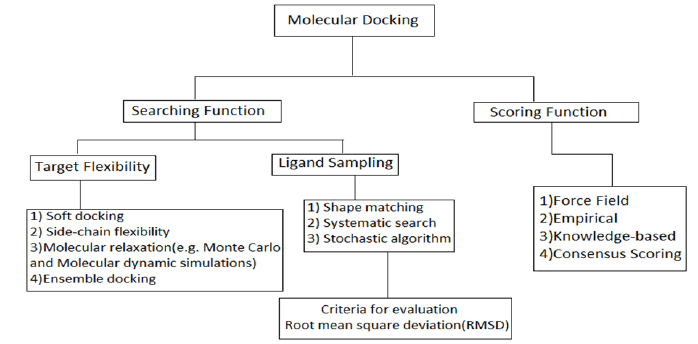
Figure 1: Methods Used for protein-ligand docking
Theory of docking
Docking is accomplished in two steps: first, sampling ligand conformations in the active site of the protein, and then ranking these conformations using a scoring function. In an ideal world, sampling algorithms would be able to replicate the experimental binding mode, and the scoring function would give it the highest score out of all created conformations. I present a brief introduction to basic docking theory from these two approaches.
Sampling algorithms: Because of the six degrees of translational and rotational flexibility, as well as the conformational degree - of - freedom of both the ligand and the protein, there is a wide range of potential binding mechanisms between two molecules. Unfortunately, computing all of the conceivable conformations would be too expensive. In molecular docking software, various sampling techniques have been developed and are frequently used.
Matching algorithms (MA) based on the molecular shape map a ligand into a protein's active site in terms of feature representation and chemical information. Pharmacophores are used to represent both the protein and the ligand. Each pharmacophore distance within the protein and ligand is determined for a match; the distance matrix between the pharmacophore and the associated ligand atoms governs the new ligand conformation. As a result of their speed, matching algorithms can be used to enhance large libraries of active compounds[3].
The ligand is placed in an active site using incremental construction (IC) procedures. By breaking the ligand's rotatable links, it is separated into many pieces, one of which is chosen to dock into the active site first. The remaining pieces can be added one at a time. The ligand's flexibility is realized by generating different orientations to fit in the active site. DOCK makes advantage of the incremental construction process.
Multiple Copy Simultaneous Search (MCSS) and LUDI are two fragment-based approaches in addition to IC. MCSS develops 1,000 to 5,000 functional group clones at the protein forcefield, which are randomly placed in the binding region of interest and subjected to simultaneous energy minimization and quenched molecular dynamics. Copies solely interact with proteins; interactions between copies are not included. Based on the interaction energies, a collection of energetically favorable binding sites and orientations for the functional group is discovered. Different functional categories are used to map the binding site. The linking of those different functional groups can be used to create new molecules that perfectly match the binding site.
The hydrogen bonds and hydrophobic interactions that potentially occur between the ligand and the protein are the focus of LUDI. Interaction locations are the core notion. A set of interaction locations is built using the rules or by scanning the database. After that, the fragment is inserted onto the interaction sites and evaluated using distance criteria. The final step is to match some or all of the fitted fragments to a single molecule.
The fitting of some or all of the conformed fragments to a single molecule is the final stage. The hydrogen bonds and hydrophobic interactions that potentially occur between the ligand and the protein are the focus of LUDI. Interaction locations are the core notion. Using the rules or scanning the database, a set of interaction sites is constructed.
Through bond rotation, rigid-body translation, or rotation, Monte Carlo (MC) procedures create ligand positions. An energy-based selection criterion is used to evaluate the conformation attained by this change. It will be stored and further adjusted to develop the next confirmation if it passes the condition. The key benefit of MC is that the change can be extremely considerable, allowing the ligand to pass the energy barriers on the potential energy surface, which is something that molecular dynamicsbased simulation approaches struggle to achieve. Examples of applying the Monte Carlo methods include an earlier version of AutoDock.
Another well-known category of stochastic approaches is genetic algorithms (GA). The GA was inspired by Darwin's theory of evolution. The ligand's degrees of freedom are represented as binary strings known as genes. These genes make up the 'chromosome,' which indicates the ligand's position. In GA, there are two types of genetic operators: mutation and crossover. Crossover swaps genes between two chromosomes, whereas mutation creates random changes to the genes. The effect of the genetic operators affecting the genes is a novel ligand structure. New structures will be evaluated using a scoring system, and those that survive (i.e., pass a threshold) will be employed in the following generation. Genetic algorithms have been used in AutoDock.
MD simulation efficiently portrays the flexibility of both the ligand and also the protein in the context of docking by shifting each atom individually in the field of the remaining atoms. However, MD simulations have the problem of progressing in extremely small increments, making it difficult to step over high-energy conformational boundaries, which can lead to insufficient sampling.
Scoring algorithms: The scoring function's goal is to distinguish between proper and inaccurate poses, or binders and inactive substances, in an acceptable amount of time. Scoring functions, on the other hand, require guessing rather than computing the protein-ligand binding affinity, and through these functions, numerous assumptions and simplifications have been used. There are three types of scoring functions: force-fieldbased, empirical, and knowledge-based scoring functions.
Hydrogen bonds, solvation, and entropy contributions are all considered in extensions of force-field-based scoring functions. I utilized software tools like DOCK and AutoDock to perform these tasks. They differ in the approach to hydrogen bonding, the structure of the energy function, and other aspects. Furthermore, the outcomes of docking with force-fieldbased parameters can be modified further using other methodologies, including linear interaction energy, to increase binding energy prediction accuracy.
Statistical analysis of ligand-protein complex crystal structures is used to calculate interatomic interaction frequencies and distances between the ligand and protein. They are based on the idea that the more advantageous an encounter is, the more probable it is to happen. Pairwise atom-type potentials are created from these frequency distributions. Within a specified cutoff, the score is derived by prioritizing favorable contacts and penalizing repulsive interactions between each atom in the ligand and protein.
Because of the restricted handling of the solvation effect, typical scoring methods have difficulty predicting affinity. Physics-based scoring is one solution to this problem.
Rigid ligand and rigid receptor docking: When the ligand and receptor are both regarded as rigid bodies only with three translational and three degrees of freedom, the search space is greatly limited. In this scenario, ligand flexibility might be addressed by allowing for a degree of atomatom overlapping between the protein and the ligand, or by using a precomputed set of ligand conformations. The ligand and receptor were kept rigid during the docking process in older DOCK versions[4].
DOCK is the world's first automated process for docking a molecule into a receptor site, and it's still evolving. It depicts the ligand and receptor assets of spheres that can be layered using a clique detection algorithm. The ligand-receptor complexes are scored using geometrical and chemical matching algorithms, and steric fit, chemical complementation, and pharmacophore similarity are all taken into account. The upgraded versions incorporate an incremental construction technique and an exhaustive search to account for ligand flexibility. The exhaustive search provides a random number of conformers that is a fraction of the ligand's spinning bonds, as specified by the user.
FLOG creates ligand conformations based on distance geometry and calculates the sets of distances using a clique finding technique. Users can identify critical sites that must be coupled with ligand atoms using FLOG. If a critical interaction is already identified before docking, this method is useful. A function that considers van der Waals, electrostatics, hydrogen bonding, and hydrophobic interaction is used to score conformations.
Flexible ligand and rigid receptor docking: As both the ligand and the receptor change conformations to form a minimum energy perfect-fit combination in processes that follow the induced fit paradigm, it's critical to consider the flexibility of both the ligand and the receptor. As a result, the most common approach is to treat the ligand as versatile while keeping the receptor rigid during docking, which is also a trade-off between precision and computational time. This methodology has been followed by almost all docking software, including AutoDock.
Moreover, the interaction of protein-protein docking could be evaluated in this version of AutoDock. AutoDock Vina was recently released as the latest version for molecular docking. FlexX uses an incremental construction algorithm to sample ligand conformations. The base fragment is first docked into the active site by matching hydrogen bond pairs and metal.
Electrostatic interactions, directional hydrogen bonds, rotational entropy, and aromatic and lipophilic interactions are all included in the present edition. The relationships between functional groups are also considered when group types and geometry are assigned.
Flexible ligand and flexible receptor docking: In the realm of docking, incorporating receptor flexibility is a big difficulty. MD simulations might theoretically model all degrees of freedom in the ligand-receptor combination.
Several theoretical models, including conformer selection and conformational induction, have been presented to illustrate the flexible ligand-protein binding process, in addition to the historical induced fit. Conformational induction explains a process in which a ligand induces a protein to adopt a conformation that it would not naturally adopt; conformer selection describes a process in which a ligand selects a favorable confirmation from several protein conformations. The easiest way for implementing receptor flexibility is "soft-docking," which reduces the van der Waals repulsion energy term in the scoring function to allow for a degree of atom-to-atom overlap between the receptor and the ligand.
Another method for understanding receptor flexibility is to use rotamer libraries. A set of side-chain conformations is included in rotamer libraries, which are normally determined through statistical analysis of structural experimental data. The use of rotamers has the advantage of sampling at a faster rate and avoiding minimization barriers.
Another method for dealing with protein flexibility is to employ an ensemble of protein conformations, which corresponds to conformer selection theory. Instead of docking a single ligand into a single stiff protein conformation, the findings are combined using one of several methods. This method was first used in DOCK, a software that calculates an ensemble's average potential energy grid and is now used in a variety of programs.
Another useful way for modeling receptor flexibility is the hybrid method. Glide, is a well-known docking application. One such example is Glide, a well-known docking application. To search all possible ligand poses and orientations within the receptor's binding area, Glide generates a series of hierarchical filters. To address ligand flexibility, a thorough search of the ligand torsion angle space is used. Torsion energies are employed to choose initial ligand conformations, and soft probabilities are used to dock them into receptor binding sites. Then, further to model receptor flexibility, a rotamer exploration is done, which is a hybrid technique that accounts for receptor flexibility by combining soft potential and multiple receptor conformations.
Ageratum conyzoides, Agerat Andrographis paniculata is a species of Andrographis paniculata (green chiretta) Momordica charantia (Bitter gourd or Bitter melon) Azadirachta indica (neem) Phyllanthus amarus has been studied and utilized as an immune stimulant, respiratory infections, anti-pathogenic bacteria, anti-HIV, anti-malaria, and fever, among other things." They are widely utilized in traditional medicine as a treatment for a variety of ailments. Phyllanthus species methanol extract was investigated for the anti-HIV-1 reverse transcriptase (RT) mechanism by inhibiting the HIV-1RT enzyme utilizing the HIV-RT assay.
I conducted a virtual screening against SARS-CoV-2 receptors to see if the bioactive constituents of Ageratum conyzoides, Azadirachta indica, Andrographis paniculata (Burm. F.) Nees, Momordica charantia, and Phyllanthus amarus, which have been used in folk medicine for various diseases, have inhibitory activity (Table 1).
Table 1: Protein-ligand Interactions Current Topics in Medicinal Chemistry.
| S. No | Name | Description | URL |
|---|---|---|---|
| Databases | |||
| 1. | ZINC | Database of commercially available compounds. It catalogs about 35 million chemically active compounds. | http://zinc.docking.org/ |
| 2. | ChEMBL | Database of compounds run by EMBL-EBI. | https://www.ebi.ac.uk/chembldb/ |
| 3. | DrugBank | A resource combining detailed drug information. | https://www.drugbank.ca/ |
| 4. | PubChem | This database of chemical compounds is maintained by the National Center for Biotechnology Information (NCBI). It also contains bioassays results. | https://pubchem.ncbi.nlm.nih.gov/ |
| 5. | GLASS | GPCR-Ligand Association database is a manually curated repository of experimentally-validated GPCR-ligand interactions. | https://zhanglab.ccmb.med.umich.edu/GLASS/ |
| 6. | Chemspider | The database is maintained by the Royal Society of Chemistry. | http://www.chemspider.com/ |
| 7. | Protein Data Bank (PDB) |
PDB contains a huge number of protein-ligand complex structures. | https://www.rcsb.org/ |
| 8. | PDBbind | The database contains information on 3,214 protein-ligand complexes. | http://sw16.im.med.umich.edu/databases/pdbbi nd/index.jsp |
| Molecular Modeling and Simulations | |||
| 9. | CHARMM | It stands for Chemistry at Harvard Macromolecular Mechanics. It is a well-known simulation program. | https://www.charmm.org/charmm/ |
| 10. | GROMACS | A well-known open-source molecular dynamics simulations program. | http://www.gromacs.org |
| 11. | Amber | It is suitable for parallel molecular dynamics simulations for larger bio-molecular systems. | https://www.ks.uiuc.edu/Research/namd/ |
| 12. | NAMD | It is suitable for parallel molecular dynamics simulations for larger bio-molecular systems. | https://www.ks.uiuc.edu/Research/namd/ |
| 13. | Desmond | It is a commercial high-performance molecular dynamics simulation program. It is incorporated into the Schrödinger program. | https://www.schrodinger.com/desmond |
| Binding Site Prediction | |||
| 14. | CASTp | Computed Atlas of Surface Topography of proteins is a program for binding site prediction. | http://sts.bioe.uic.edu/castp/ |
| 15. | CAVER | A tool for analysis and visualization of tunnels and channels in protein structures. | http://www.caver.cz/ |
| Docking and Screening | |||
| 16. | AutoDock4/Vina | Developed by Scripps Research Institute both Auto Dock 4 and Auto Dock Vina are widely used open-source docking programs. | http://autodock.scripps.edu/ |
| 17. | DOCK | Open source docking program for academic purposes. | http://dock.compbio.ucsf.edu/ |
| 18. | GOLD | Commercially available screening and docking program. | https://www.ccdc.cam.ac.uk/solutions/csd discovery/components/gold/ |
| 19. | Swiss Dock | It is an open-source web server for molecular docking governed by the Swiss Institute of Bioinformatics. | http://www.swissdock.ch/ |
| 20. | GLIDE | Commercially available docking program. It is a part of the Schrödinger package. | https://www.schrodinger.com/glide/ |
| 21. | Pharmer | Open source pharmacophore-based screening program. | http://smoothdock.ccbb.pitt.edu/pharmer/ |
| 22. | CATALYST | Pharmacophore design and analysis program. It is a part of the Discovery Studio suite. | http://www.3dsbiovia.com/products/collaborati ve-science/bio via-discovery studio/pharmacophore-and-ligand-based design.html |
| 23. | LiSiCA | Ligand Similarity using Clique Algorithm is a ligand-based virtual screening program. | http://insilab.org/lisica/ |
| 24. | Ligand Scout | It is a commercial program for 3D pharmacophore designing and virtual screening. | http://www.inteligand.com/ligandscout/ |
| 25. | PyRx | Both open-source and commercial programs for virtual screening. | https://pyrx.sourceforge.io/ |
| 26. | Swiss Similarity | Web server for rapid ligand-based virtual screening. | http://www.swisssimilarity.ch/ |
| 27. | ZINCPharmer | ZINCPharmer is a free pharmacophore search server for screening the purchasable subset of the ZINC database. | http://zincpharmer.csb.pitt.edu/ |
| Target Prediction and Ligand Design | |||
| 28. | Patch search | It is an R package for target prediction. | https://github.com/MTiPatchSearch/patchsearch |
| 29. | Swiss Target Prediction | Web server for drug target prediction. | http://www.swisstargetprediction.ch/ |
| 30. | SEA | The Similarity ensemble approach relates proteins based on the set-wise chemical similarity among their ligands. | http://sea.bkslab.org/ |
| 31. | GANDI | This is a program for structure-based fragment-based ab initio ligand design. | http://www.biochem-caflisch.uzh.ch/download/ |
| 32. | LUDI | Automated program for structure-based ligand design. It comes incorporated in the Discovery Studio suite. | http://www.3dsbiovia.com/products/collaborati ve-science/biovia-discovery-studio/ |
| Binding Affinity and Free Energy Prediction | |||
| 33. | Hyde | Commercial program for binding affinity prediction. | https://www.biosolveit.de/Hyde/ |
| 34. | X-Score | Open source program for binding affinity scoring. | http://sw16.im.med.umich.edu/software/xtool/ |
| 35. | DSX Online | This program serves as a user interface for the knowledge-based scoring functions DSX. | http://pc1664.pharmazie.uni marburg.de/drugscore/ |
| QSAR | |||
| 36. | CQSAR Package | This contains a dual database of over 21,000 QSAR models. | http://www.biobyte.com/bb/prod/cqsar.html |
| 37. | QSAR Pro | Commercial suite for QSAR modeling and activity prediction. | http://www.vlifesciences.com/products/QSARP ro/Product_QSARpro.php |
| ADMET Property Prediction | |||
| 38. | Swiss ADME | Web server for ADME property analysis. | http://www.swissadme.ch/ |
Ligand preparation
PubChem was used to extract 198 chemicals from 5 different plants in SDF format. The bioactivities of several of these ligands, which are essentially bioactive molecules in medicinal plants, were retrieved. In Spartan '14 version 1.1.4, the ligands were opened and turned to a 3D structure. Using the Semi-Empirical technique and a basic set of PM3, the ligands were energetically reduced and geometrically optimized. Lipinski's filter and Veber's guidelines for oral bioavailability of drug candidates were used to computing druglike characteristics. s (500g/mol), clog P (5%), Hydrogen bond acceptors (5%), hydrogen bond donors (10%), and molar refractivity are all filter characteristics[5].
Receptor preparation
SARS-CoV-2, COVID-19, and spike glycoprotein crystal structures were obtained in PDB format from the RCSB Protein Data Bank. Using warez.2020's Discovery Studios, all water molecules, and heteroatoms were removed from the crystal structures, which were then recorded in PDB format. The docking research for the spike glycoprotein (6VXX) utilized the alpha–chain (A chain). This is because the amino acid sequences have numerous alignments, which means they have identical residues. The docking evaluation findings were analyzed using the amino acid composition of the active site (Figure 2) .
Figure 2: Process of Molecular Docking.
The receptors (PDB ID: 6LU7, 6LZG, 6VXX) were treated, which included the deletion of water molecules, ligand, and heteroatoms. For protein remodeling and the insertion of the polar hydrogen group, AutoDock version 4.2.6 was employed. The preparation of the receptor previous to docking, ligands, the grid box, and the algorithm is all part of virtual screening using automated docking correct grid box size for the probable binding site of the ligands was analyzed by PyRx and AutoDock Vina, and the receptor grid center was set on the receptor's active site residue. The docking analyses were performed using AutoDock4.2.6, Pymol, and Discovery Studio. The docked complexes were analyzed to find compounds with higher binding energy and lower ligand inhibitory constant (Ki) with the target protein. The DiscoveryStudio2020 application was used to analyze the structural complexity of the ligand-protein interaction. The top three categories of compounds in the docking investigations were submitted to molecular dynamics (MD) simulations based on their binding affinities in complexation with the major protease and the two spike glycoproteins of SARS-CoV-2. The needed counter ions were used to neutralize the ligandprotein complexes. The complexes were then solvated with a TIP3P water box of 12 after that. For MD simulations, the solvated parameters were saved[6-7].
Expected outcome
This study looked at 33 active compounds in medicinal plants that were utilized as antioxidants or antivirals and passed the Lipinski thumb criterion, as well as seven additional compounds. Two hundred and eight (208) compounds were docked in total. Ivermectin, Friedelin, Diosgenin, Nimolicinol, Multiflorenol, Meliantriol, Taraxerol, Momordicoside-I, Azadiradione, Beta-amyrin, Nimolinone, Nimbaflavone, Epox yazadiradione, Momordicin, Astragalin, Nimbinin, and Epiazadiradione were discovered to have a strong relationship. The essential residue(s) for each ligand's hydrogen bond interactions with the SARS-CoV-2 receptors, as well as their corresponding distances Proteins, require hydrogen bonding interactions because they provide the organization for unique folding and selectivity for molecular recognition at the ligand-protein interface. The top three drugs, astragalin, nimbaflavone, and kaempferol, were studied using molecular dynamics simulations in association with SARS-major CoV-2's protease. Astragalin has been shown to have antiviral properties as well as anti-H1N1 influenza virus activity Astragalin has been shown in studies to be an efficient lead chemical in combating oxidative stress caused by endotoxin, which causes airway dysfunction and inflammation. The study used cutting-edge virtual screening and molecular dynamics simulations, which are critical approaches for drug creation and finding novel inhibitors [8].
[Google Scholar] [ Crossref]
Citation: Chauhan K. Molecular Docking of Medicinal plants compounds as new potential inhibitors of novel coronavirus. Int J Inn Res Sci Eng Tech, 2022, 3(5), 055-060.
Received: 29-May-2022, Manuscript No. IJIRSET-22-65359; Editor assigned: 30-May-2022, Pre QC No. IJIRSET-22-65359(PQ); Reviewed: 04-Jun-2022, QC No. IJIRSET-22-65359(Q; Revised: 06-Jun-2022, Manuscript No. IJIRSET-22- 65359(R); Published: 10-Jun-2022, DOI: 10.35248/ijirset.22.3(5).55-60.
Copyright: 2022 Chauhan K. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.