Case Report - (2019) Volume 8, Issue 4
Metastatic neuroblastoma with opsoclonus raccoon eye presentation is an extremely rare condition presented in stage IV metastatic condition. Histopathology/ cytology, vanillylmandelic acid (VMA), homovanillic acid (HVA) levels and metaiodobenzylguanidine (MIBG) scanning are mainly contributors for the diagnosis of this disease. We are presenting one year male child with history of protrusion of right eye, swelling in right periauricular region, opsoclonus movements of small amplitude and periorbital ecchymosis ‘raccoon eye’. So far only infinitesimal cases has been reported in the literature and our present case report is an extremely rare case of locally advanced metastatic neuroblastoma, who presented as opsoclonus raccoon eye and despite of the aggressive chemotherapy, didn’t responded to the intended treatment. We attempt a review in the literature for different possibilities of staging and diagnostic management.<
Opsoclonus, Raccoon eye, Metastatic neuroblastoma, Vanillylmandelic Acid (VMA), Homovanillic Acid (HVA)
Neuroblastoma is an enigmatic malignant neoplasm. In its early stages it can be readily cured with surgery or, in some circumstances, can even spontaneously regress or mature to a benign ganglioneuroma. In the more common advanced stages, the disease is often fatal. It is one of the first malignancies in which molecular biologic assays have influenced treatment and prognosis. The three major cardinal eye signs of neuroblastoma include proptosis, Horner's syndrome and opsoclonus eye movements. Treatment of neuroblastoma involves chemotherapy, surgery, radiation and autologous bone marrow transplantation, which is used as a rescue following marrow-ablative induction chemotherapy, while the patients with ophthalmic involvement requires aggressive multiagent high doses chemotherapy.
A one year male child, presented with a history of protrusion of right eye since three months. Child was born out of full term normal vaginal delivery at home with normal cry at birth. His feeding and stool habits were normal. All milestones were achieved till the age of 9-months. At this age, he developed a swelling over the right eye, which was gradually progressive in size and associated with excessive crying. Local examination revealed a swelling in right periauricular region of 6 × 5 cm in size reaching up to right fronto-temporal region (Figure 1).
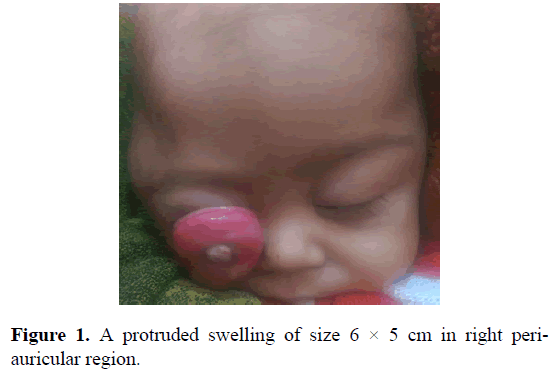
Figure 1. A protruded swelling of size 6 × 5 cm in right periauricular region.
It was firm to hard, shining, translucent mass, fixed to the underlying structures. The right eye had rapid, involuntary, multivectorial, unpredictable, conjugate fast eye movements without intersaccadic intervals. The movements of opsoclonus were small in amplitude and with little deviations from the primary position. He also had severe circumciliary and deep ciliary congestion of the right eye and had severe photosensitivity towards the light.
Patients presented with elevated levels of vanillylmandelic acid (VMA) and homovanillic acid (HVA) measured by high-performance liquid chromatography. There was no history of any other swelling in the body. There was no history evident of any malignancy in the family. Mother of the child had one abortion at 8-months of pregnancy and one normal female child of 4-years age. There was no significant past, medical or surgical history. General physical and systemic examination was normal. Complete hemogram and routine blood biochemistry parameters of the patient were within normal limits.
Patient underwent contrast enhanced computed tomography (CECT) of the head and neck region, which revealed multiple soft tissue enhancing lesions seen in between cranial sutures extending in the orbits bilaterally with erosions of underlying bone with possibility of intrasutural metastasis. There was hyperdense lesion seen in left lateral ventricle with significant midline shift towards left side. CECT also revealed fracture of the occipital bone.
CECT abdomen revealed heterogeneously enhancing mass of size 9.8 cm × 5.7 cm × 9.1 cm with internal necrotic areas and multiple foci of calcification seen in left suprarenal region extending to retroperitoneum from L1 vertebral body to the upper aspect of sacrum, crossing midline towards right. There were irregular lytic areas with destruction along the posterior margin of the vertebral body seen from L2-L4 vertebral bodies, which was possibly due to lumbar vertebral metastasis. The lesion was encasing the aorta and displacing the inferior vena cava (IVC) right-laterally and left kidney inferiorly. The lesion was causing the mass effect over bilateral kidney (left>right) with dilatation of left renal pelvis due to mass effect over the left ureter. Right testis was also showing heterogeneous enhancement and appears bulky as compared to left side suggestive possibility of testicular metastasis (Figure 2).
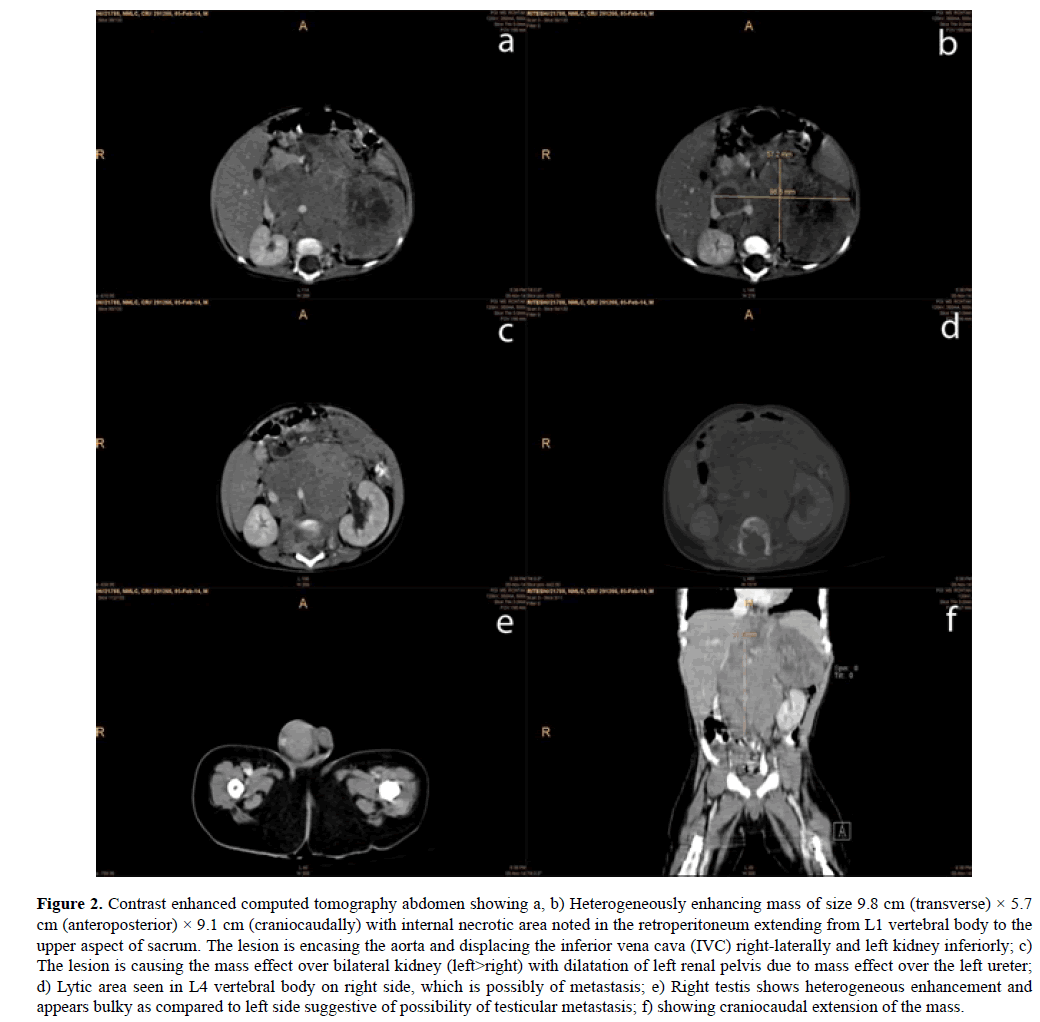
Figure 2. Contrast enhanced computed tomography abdomen showing a, b) Heterogeneously enhancing mass of size 9.8 cm (transverse) × 5.7 cm (anteroposterior) × 9.1 cm (craniocaudally) with internal necrotic area noted in the retroperitoneum extending from L1 vertebral body to the upper aspect of sacrum. The lesion is encasing the aorta and displacing the inferior vena cava (IVC) right-laterally and left kidney inferiorly; c) The lesion is causing the mass effect over bilateral kidney (left>right) with dilatation of left renal pelvis due to mass effect over the left ureter; d) Lytic area seen in L4 vertebral body on right side, which is possibly of metastasis; e) Right testis shows heterogeneous enhancement and appears bulky as compared to left side suggestive of possibility of testicular metastasis; f) showing craniocaudal extension of the mass.
Cytopathology of the abdominal mass revealed mitotic figures with psuedorosettes presentation of the tumor cells (Figure 3). Cytopathological findings from the lytic area, orbital involvement and related clinical presentation dragged the diagnosis towards Stage-IV metastatic neuroblastoma.
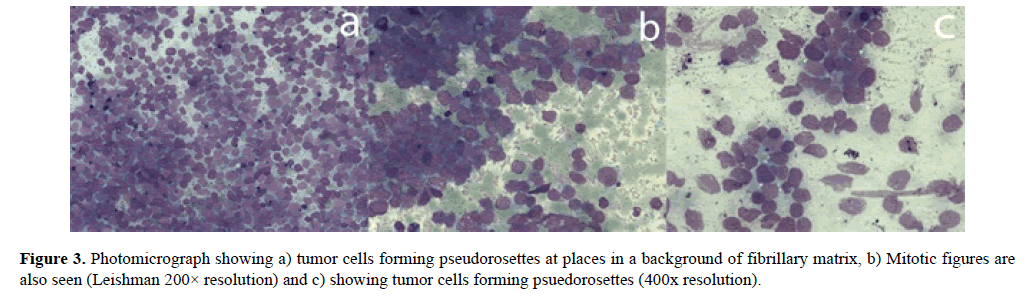
Figure 3. Photomicrograph showing a) tumor cells forming pseudorosettes at places in a background of fibrillary matrix, b) Mitotic figures are also seen (Leishman 200× resolution) and c) showing tumor cells forming psuedorosettes (400x resolution).
Patient underwent ultrasonography of the abdomen which revealed a large heteroechoic lesion with foci of calcification measuring 12.0 × 9.0 × 9.0 cm seen in retroperitoneum encasing aorta, crossing midline and showing flow on color doppler and a heteroechoic lesion of size 6.0 cm × 4.0 cm seen in left suprarenal region with foci of calcification. Multiple hypoechoic lymph nodes were seen in para-aortic region with obscured pancreas.
The poor general condition of the patient as well as progressive nature of the locally advanced disease prompted us to treat the patient with palliative intention. Patient was treated with six cycles of 3-weekly intravenous combination chemotherapy with vincristine 0.5 mg, doxorubicin 15 mg & cyclophosphamide 200 mg. Patient was having good palliation and subsequently decided for second line chemotherapy, but he lost to follow-up.
Neuroblastoma is the most common extracranial solid tumor of childhood which accounts for 8-10% of all pediatric cancers [1]. The most common clinical presentation is that of an abdominal mass and majority of the patients (>90%) are diagnosed before the age of 10-years, while up to 50% of patients presenting with metastatic disease at the time of diagnosis [1]. The median age at diagnosis is 2-years. Neuroblastic tumors are derived from primordial neural crest cells and ultimately populate the sympathetic ganglia, adrenal medulla and other sites [2]. Recent advancements in the understanding of tumor biology have aided the diagnostic management of this disease. Pain is the most common presenting symptom. This frequently is caused by bone, liver, or bone marrow metastases or local visceral invasion by the primary tumor. Tumor mass is mainly responsible for other constitutional symptoms, which may include weight loss, anorexia, malaise and fever. Respiratory distress may accompany massive hepatomegaly, especially in infants with stage IV-S disease. Skin metastases may have a bluish tinge, giving the classic “blueberry muffin” sign, however, as in the present case, patients with widespread metastasis, extensive bone marrow involvement may result in pancytopenia and therefore presents with subconjunctival hemorrhages. Paraneoplastic neurologic findings including cerebellar ataxia or opsoclonus/ myoclonus (dancing eyes, dancing feet) may occur. Retrospective reviews have found that about 10-20% of cases have orbital metastases [3]. Ophthalmic manifestations are well documented, which ranges from symptom-free metastasis picked up on imaging to vision threatening orbital involvement and includes proptosis, periorbital ecchymosis, Horner syndrome (oculosympathetic palsy), opsoclonus/myoclonus ocular motility defects, ptosis and blindness [3,4].
The three major eye signs of neuroblastoma, proptosis, Horner's syndrome and opsoclonus, are closely related to the site, stage of tumor and outcome of the patient. As in our present case, orbital metastatic neuroblastoma, presenting with proptosis and periorbital ecchymosis (raccoon eyes), is considered one of the classic signs of metastatic neuroblastoma in children [2]. Horner syndrome and opsoclonus are infrequent manifestations of neuroblastic tumor mass effect on the sympathetic innervation of the eye [5]. Horner syndrome is associated with localized neuroblastoma and consequently demonstrates markedly better survival rates than those seen in cases of orbital metastasis. Various gene markers are evolving as useful prognostic and resolution indicators [6]. The most important prognostic factor is age at diagnosis; early diagnosis, when the tumor is still localized and surgically resectable, is second in importance [2].
Bone marrow aspirate and biopsy frequently show metastatic tumor deposits that can establish the diagnosis. Pathologic evaluation of bone marrow is also a requirement for staging of neuroblastoma. As seen in present case also, characteristically, the cytological findings of neuroblastoma appear in clumps and pseudorosettes. The absence of pseudorosettes does not eliminate the possibility of neuroblastoma. Either HVA or VMA, metabolites of dopa/norepinephrine and epinephrine, respectively, is elevated in >90% of patients with stage IV neuroblastoma [2,7,8].
A ratio of VMA to HVA of >1.5 is associated with a favorable prognosis in patients with metastatic neuroblastoma. The most commonly used staging system is the International Neuroblastoma Staging System (INSS). It is based on clinical, radiographic and surgical findings. Treatment of the primary disease is based on Children's Oncology Group Neuroblastoma Risk Group Assignment Schema of low, intermediate or high [6]. Orbital involvement falls in the high-risk category [3]. According to the International Neuroblastoma Staging System, orbital involvement is a sign of stage IV disease [2]. Patients with localized disease (Stages I & II) have a better prognosis than those with neuroblastoma that has metastasized to distant sites (Stage IV), including bone and bone marrow [1]. Other features associated with poor prognosis are unfavorable histology, amplification of the MYCN oncogene, characteristic chromosomal losses (1p) and gains (17q), age older than one year, and elevated ferritin and urinary catecholamine levels. Important imaging studies for staging include CECT and MRI, as well as meta-iodobenzylguanidine (MIBG) scanning [1]. Preferentially taken up by adrenergic secretory vesicles present in neuroblastoma (and adrenal medullary) cells, MIBG has proven to be a sensitive technique to identify sites of metastatic disease and to evaluate response to treatment [1].
Treatment of neuroblastoma involves chemotherapy, surgery, radiation and autologous bone marrow transplantation, which is used as a rescue following marrow-ablative induction chemotherapy. Treatment of patients with ophthalmic involvement involves aggressive multi-agent chemotherapy consisting of very high doses. After a response to chemotherapy, resection of the primary tumor should be attempted, followed by myeloablative chemotherapy. Radiation therapy to the primary site may be indicated in high-risk patients [3]. In an attempt to improve the outcome, recent therapeutic advances include the use of differentiation agents such as 13-cis-retinoic acid for a period of 6- months [1]. It is thought that retinoic acid causes decreased proliferation, decreased expression of the MYCN oncogene and morphologic differentiation to mature, non-dividing cells. Both myeloablative therapy and retinoic acid improve outcome in patients categorized as high risk [1]. In addition, immunotherapy using the murine 3F8 antibody (directed against the GD2 surface antigen present on neuroblastoma cells) is undergoing experimental evaluation [1]. Preliminary studies indicate feasibility and efficacy of this novel treatment modality. Finally, 131I-MIBG has been used to selectively target radiation to residual or recurrent neuroblastoma in association with myeloablative chemotherapy and hematopoietic stem cell rescue [6]. Although promising, the impact of these novel therapies on the long-term survival of patients with Stage-IV neuroblastoma remains to be determined.
Published reports of 3 or 5-year survival rates in patients with stage IV disease show significant improvement in outcomes in recent years, reflecting improved efficacy of treatment regimens [7]. Patients with localized disease have a 95% cure rate; those with intermediate stage neuroblastoma have a 70-80% cure rate; those with advanced disease only have 20-30% cure rate. Survival rates for patients diagnosed with stage IV disease after 1- year of age range from 2.5% mid-century to 16% in the 1980s and 38% in the 1990s [2,3]. Comparative data from the SEER program reports a 5-year survival of 90% for those diagnosed at less than 1-year, while only 66% of those diagnosed between the ages of 1 through 4 years achieved a 5-year survival [8].
Differential diagnosis
In addition to neuroblastoma, differential diagnosis includes Non-Hodgkin lymphoma, rhabdomyosarcoma, Ewing’s sarcoma, primitive neuroectodermal tumor (PNET), and esthesioneuroblastoma. Non-Hodgkin lymphoma and rhabdomyosarcoma involving the orbit or paranasal sinuses can present clinically in children as acute bilateral blindness [9]. Ewing sarcoma/PNET has been reported as a primary neoplasm of skull bones and sinuses and also as a metastatic lesion [10]. A primary Ewing sarcoma/PNET of the orbit has been associated with unilateral visual loss. Esthesioneuroblastoma is a much less common tumor, especially in young children, and typically presents with a different set of symptoms, but presentation with sudden visual loss has been described [11].
Histopathological evidence of neuroectodermal differentiation argues against Non-Hodgkin lymphoma and rhabdomyosarcoma. Neuroblastoma and Ewing sarcoma/PNET can show similar morphology. Immunohistochemical studies of PGP9.5 and MIC-2 expression are very helpful in the diagnosis of neuroblastoma [11].
Ophthalmic involvement in neuroblastoma is uncommon and has an ominous prognosis. Different investigations like bone marrow aspiration cytology, vanillylmandelic acid (VMA), homovanillic acid (HVA) levels and meta-iodobenzylguanidine (MIBG) scanning should be adopted in practice to diagnose at the early stage. The clinical management presents considerable challenges. Intensive multimodality approach should be tested, incorporating surgery, dose intensive combination chemotherapy etc. It is the first malignancies in which molecular biologic assays have influenced treatment and prognosis, hence future challenges include the development of less toxic therapy for patient with localized disease and new approaches for patients with metastatic disease for the treatment or palliation.
Consent was obtained from the patient for publication.
There is no conflict of interests to declare from any of the authors regarding this paper.
We are thankful for our Departmental team for their help in collection of data and in this review.
Received: 23-Nov-2018
Copyright: © 2019 Anil Kumar Dhull et al. This is an open access paper distributed under the Creative Commons Attribution License. Journal of Biology and Today's World is published by Lexis Publisher; Journal p-ISSN 2476-5376; Journal e-ISSN 2322-3308.