Research Article - (2022) Volume 11, Issue 5
Background: This investigational study reports initial results from testing a new type of mechanical thrombectomy device in a porcine model. The novel technical innovation involves the use of two manually expandable wire spheres that are capable of opening between 3-16 mm in diameter independently of one another and moving independently of one another fore and aft along a common shaft over a 5 cm distance.
Methods: The investigational thrombectomy device was introduced and deployed in vivo within the porcine venous and arterial vasculature. Following deployment and device activation within the animal’s vascular system, arteries and veins were surgically explanted and histopathologic examination was conducted to determine the device’s effects on the vascular surfaces. Statistical analysis was not utilized.
Results: Repeated device deployment and use in porcine veins and arteries failed to demonstrate intimal injury (radiographically and histologically) even when the device’s spheres were expanded beyond 100% of the native vessel’s diameter and translated along the vessel’s intima.
Conclusion: This in vivo study of a novel mechanical thrombectomy device has demonstrated no untoward effects on the internal anatomy of instrumented arteries and veins despite active device expansion repeated surface wall fore and aft friction. Future investigations aim to demonstrate that this device can better treat acute, subacute and chronic venous and arterial thromboembolic disease as compared to currently available technologie
Trial Registration: This article does not report the results of a health care intervention on human participants.
Thrombectomy • Mechanical • Venous • Arterial
PA: Pulmonary Artery; CA: Carotid Artery; IJV: Internal Jugular Vein; JV: Jugular Vein; CIV: Common Iliac Vein; PE: Pulmonary Embolus
In the United States, venous and arterial thrombotic disease affects >700,000 individuals/year. Treatment protocols include combinations of conservative therapy, antiplatelet/antithrombotic medication administration, thrombolysis with or without intravascular ultrasound, angioplasty, luminal stenting, suction thrombectomy and/or mechanical thrombectomy [1-7]. These approaches have varying degrees of revascularization success depending upon targeted anatomic location and thrombus character. While acute clot is quite responsive to a variety of treatment modalities, subacute and chronic clot is often more difficult to clear due to its firm and fibrous characteristics and vessel wall adherence. To make matters more difficult, endovascular surgeons commonly cannot predict the thrombus’ quality/character prior to initiating intervention. This often means a single approach fails or several different devices are needed to successfully revascularize a particular artery or vein. The consequences of failure are self- evident while the need to use multiple different devices increases procedure duration, expense and associated blood loss.
To address the above shortcomings of current thrombectomy/revascularization techniques. Retriever Medical, Inc. has developed a patented (US 10,172,634 B1; US 10,258,357 B1; US 10,898,215 B2; 11,382,643 B2) novel percutaneously introduced thrombectomy device that simultaneously and atraumatically utilizes a combination of aspiration, mechanical expansion and linear translation to remove acute, subacute and chronic thrombus from arteries and veins while limiting blood loss and eliminating the need for thrombolytic infusion. As part of this development process, the first and second generation devices’ immediate effects on the virgin arterial and venous vascular endothelial lining as well as its ability to evacuate thrombus from the pulmonary arterial tree has been evaluated in vivo using a live porcine model. The results of these studies are reported below.
Retriever Medical’s thrombectomy device, ClotHound™, is displayed in Figure 1. This percutaneously introduced technology incorporates manually expandable nitinol spheres that can be control expanded, independent of one another, along a diameter range from 3 mm-16 mm. The proximal sphere can also be linearly translated 5 cm fore and aft along the device’s shaft, thus permitting curettage, mobilization, and capture of acute, subacute, and chronic thrombus. The distal sphere is fixed to the device and as such can function to capture thrombus, remove thrombus, anchor the device in place and provide distal embolic protection. The spheres are manipulated using the proximal handle with its integrated sliding tabs. Depending upon the vessel being accessed, physicians can select between pre-shaped 24F and 20F guide and delivery/aspiration catheters.
Figure 1: ClotHound Mechanical Thrombectomy Device.
Note: (A) Proximal sliding manually expandable sphere; (B) Distal non-sliding expandable sphere; (C) Control handle with sliding activating tabs; (D) Aspiration catheter; (E) Aspiration syringe; (F) Orange Arrow points to the mechanical device’s delivery catheter.
The Retriever ClotHound thrombectomy system device differs from currently available devices in several ways:
The device’s analysis was divided into two studies. The vessels tested in both studies included the swine IJV, JV, EJV, CIV, CA and PA. Aside from the CA, these vessels’ walls are thinner and weaker than that of the PA due to the blood pressure they are each designed to carry (0 mm-20 mm Hg for IJ and JV vs 25 mm-90 mm Hg for PA). Given this factor, device testing against the thinner IJV and JV vessel wall is expected to represent a sensitive test when evaluating risk of vessel wall and intimal device mediated injury. In comparison to the IJV and JV, the PA, should be more tolerant of equivalent radial wall forces.
Study #1
Two swine were utilized to investigate the acute effects of the first generation ClotHound mechanical thrombectomy device and associated catheters on the venous wall and intimal lining. Each animal was placed under general anesthesia. Venous access was obtained via femoral cut down and sheath placement. All procedures were performed after IRB approval (Study ID #:NSG07123N).
In the first animal (85 lbs.), the ClotHound was used to curettage the right IJV, the left IJV, the right EJV, the left EJV and the right CIV under known radial force pressures. Prior to instrumentation, an angiographic measurement of the Internal Diameter (ID) of each vessel was calculated and the sphere was subsequently expanded 40% greater than the ID of each vessel. The radial force on the vessel wall was calculated by comparing a measured force at the device handle to radial force data previously collected in a controlled bench top environment. Once the device was expanded 40% greater than the vessels measured ID, it was linearly advanced fore and aft five times over a 3 cm length in each vessel to evaluate its effects on the vessel endothelium.
In order to test the device’s ability to a traumatically evacuate an acute PE, a robust a cellular clot predicate was endovascular placed into the PA of two swine (85 lbs and 115 lbs) using a model devised by Schultz, et al. [8]. Once an arteriogram confirmed clot burden in the left lower lobe branch of the PA, a femoral approach was used to advance an Amplatz guidewire into the PA and through the clot predicate. For purposes of this study, a 16 French Aspiration Catheter was placed over the wire and positioned proximal to the predicate. The mechanical thrombectomy device, sheathed in its delivery catheter, was advanced into the clot predicate and the distal and proximal spheres were expanded and used to mobilize the thrombus.
Study #2
In Study #2, two swine were subjected to both venous and arterial testing using the same methodology described in Study#1. In these animals, however, a second generation ClotHound thrombectomy device that was at the design freeze level was utilized to determine if minor changes to the first-generation device had impacted venous and arterial intima in a negative fashion. To stress the system, device expansion was carried out at both native vessel diameter and 20% greater than native diameter to stress the vessel’s wall integrity. As in Study #1, instrumented vessel segments were acutely explanted for histologic evaluation. All procedures were performed after IRB approval (Study ID #:NSG07123N).
All animals were euthanized immediately after testing and the instrumented segments of vessels were surgically harvested and evaluated. H&E staining of venous cross sections was performed by a veterinary pathologist (Table 1). In both Studies 1 and 2, post device manipulation angiograms demonstrated no significant venous/arterial intimal damage thus suggesting that manipulation of the over expanded intraluminal sphere against vessel wall was structurally non-disruptive.
| Vessel | Pathology Evaluation |
|---|---|
| #1 Left Iliac Vein | Linear tangential section of blood vessel: Normal vasculature (CONTROL VESSEL) |
| #2 Right Iliac Vein | Linear tangential sections of blood vessel: The several sections of tangential blood vessels appear normal with post-mortem pooling. Scattered loss of endothelial lining in some of the vessels (EXPERIMENTAL VESSEL) |
| #3 Left Ex Jugular Vein | Linear tangential sections of blood vessel: The several sections of tangential blood vessels appear normal with post-mortem pooling: (EXPERIMENTAL VESSEL) |
| #4 Left Int Jugular Vein | Linear tangential sections of blood vessel: The several sections of tangential blood vessels appear normal with post-mortem pooling. Scattered loss of endothelial lining in some of the vessels (EXPERIMENTAL VESSEL) |
| #5 Right Ext Jugular Vein | Linear tangential sections of blood vessel: The several sections of tangential blood vessels appear normal and are expanded by pooling blood and early fibrin clot formation. Scattered loss of endothelial lining in some of the vessels (EXPERIMENTAL VESSEL) |
| #6 Right In Jugular Vein | Linear tangential sections of blood vessel: The several sections of tangential blood vessels appear normal with some pooling of blood and early fibrin clot formation. Scattered loss of endothelial lining in some of the vessels (EXPERIMENTAL VESSEL) |
Table 1. Pathology Evaluation Summary: In all specimens, the venous vascular integrity was intact, and the histology of vascular elements was essentially normal. There was no definitive pathology associated with the instrumented veins when compared to no instrumented control vessels.
Once clot predicate was mechanically removed by the device, angiography documented successful thrombectomy without PA damage. In the first animal, post procedural angiography revealed complete clot removal without associated vessel damage thus indicating that intraluminal device manipulation was safe. In the second animal, an estimated 80% of the clot predicate was removed after the first pass while a second pass removed the remaining clot. Angiography again revealed that thrombus extraction was a traumatic (Figure 2).
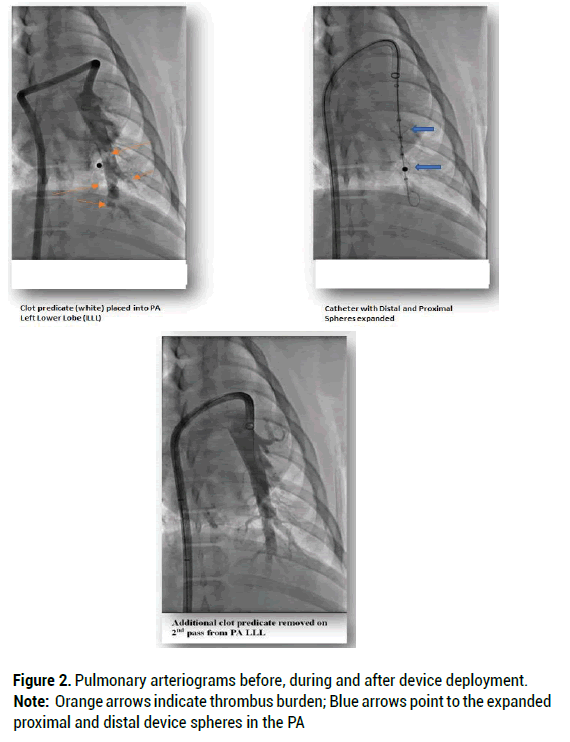
Figure 2: Pulmonary arteriograms before, during and after device deployment.
Note: Orange arrows indicate thrombus burden; Blue arrows point to the expanded proximal and distal device spheres in the PA
In all specimens, the vascular wall integrity was intact, and the histology of vascular elements remained essentially normal. In some specimens there was art factual disruption of the vascular endothelium characterized by localized loss of endothelium and fragmentation of the vascular wall, without associated inflammation or hemorrhage. More specifically, device manipulation within the lumen of the evaluated vascular structures did not appear to cause significant structural damage to the integrity of the blood vessel or cause significant inflammatory response. Figures 3-7 provide H and E micrographs of instrumented vessels.
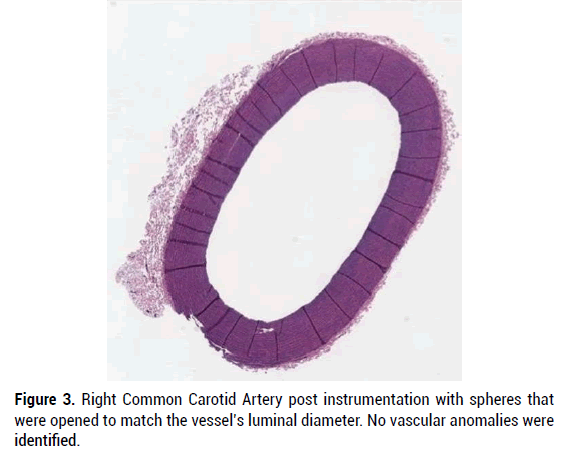
Figure 3: Right Common Carotid Artery post instrumentation with spheres that were opened to match the vessel’s luminal diameter. No vascular anomalies were identified.
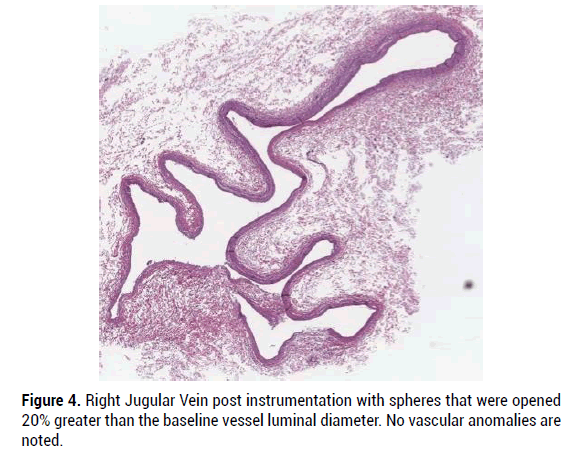
Figure 4: Right Jugular Vein post instrumentation with spheres that were opened 20% greater than the baseline vessel luminal diameter. No vascular anomalies are noted.
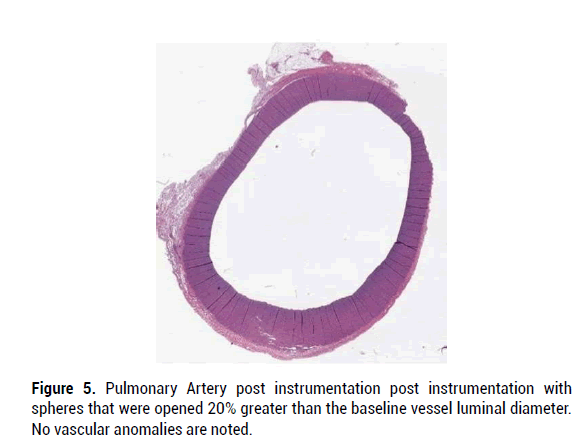
Figure 5: Pulmonary Artery post instrumentation post instrumentation with spheres that were opened 20% greater than the baseline vessel luminal diameter. No vascular anomalies are noted.
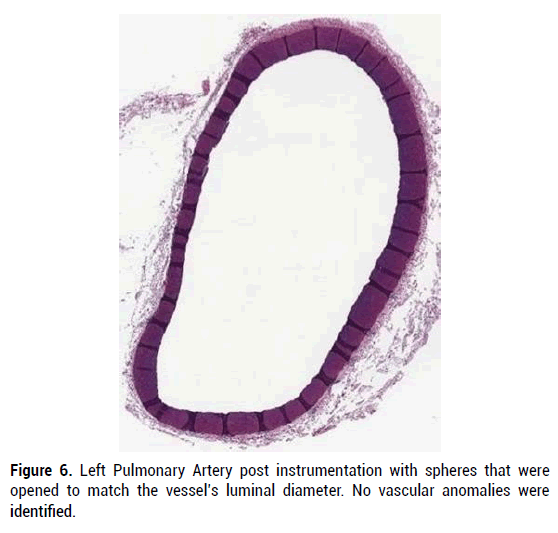
Figure 6: Left Pulmonary Artery post instrumentation with spheres that were opened to match the vessel’s luminal diameter. No vascular anomalies were identified.
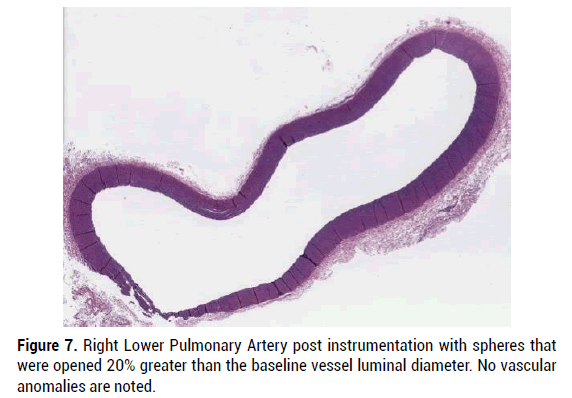
Figure 7: Right Lower Pulmonary Artery post instrumentation with spheres that were opened 20% greater than the baseline vessel luminal diameter. No vascular anomalies are noted.
Together, these two studies support that expansion of the Retriever ClotHound thrombectomy spheres 20-40% greater than the baseline vessel diameter can be performed without significant deleterious effects to the targeted and instrumented arteries and veins. Five cycles of 3 cm-5 cm translational fore and aft movement of the proximal expanded sphere also failed to significantly injure any tested vessel.
Based on these findings, this data can be used to support safe expansion and fore and aft movement of the spheres within peripheral veins, pulmonary arteries, and peripheral arteries for the treatment of human veno/arterial thrombotic disease. These studies also indicate that the ClotHound can effectively and a traumatically remove thrombus from the pulmonary vein and the peripheral venous/arterial tree in a swine model. This study is an initial proof of concept of safety using a porcine model. Additional studies are required to determine safety and efficacy in humans.
Ethics approval and consent to participate
This study was performed at the University of Buffalo, Buffalo, New York under IRB approval (ID#: NSG07123N). All animals were treated per University of Buffalo the ethical guidelines for the use of living creatures. This study included no human data.
Consent for publication
No individual personal data was contained in this study.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request. All data generated or analysed during this study are included in this published article.
Competing interests
Michael Horowitz, MD (MH) is a device inventor, Founder of Retriever Medical and Board member of Retriever Medical. He holds equity in Retriever Medical. Brandon Repko, MD (BR) is a device inventor and Consultant for Retriever Medical. He holds equity in Retriever Medical. Hieu Le (HL) is Vice President of Retriever Medical. He holds equity in Retriever Medical. Benjamin Bobo (BB) is a device inventor and CEO of Retriever Medical. He holds equity in Retriever Medical. Julia Bulova (JB) has no conflicts of interest.
Funding
This study was designed and funded by Retriever Medical, Inc. Histologic analysis of biologic material was performed by an independent contractor. Study design and data collection was performed by MH, BR, HL and BB.
Author contributions
Study design: MH, BB, HL, BR
Procedure performance: BR
Manuscript preparation: MH, JB
[Cross ref] [Google scholar] [PubMed]
[Cross ref] [Google scholar] [PubMed]
[Cross ref] [Google scholar] [PubMed]
[Cross ref] [Google scholar] [PubMed]
[Cross ref] [Google scholar] [PubMed]
[Cross ref] [Google scholar] [PubMed]
[Cross ref] [Google scholar] [PubMed]
[Cross ref] [Google scholar] [PubMed]
Citation: Horowitz MB, et al. Porcine Testing of a Novel Second Generation Mechanical Thrombectomy Device to Evaluate Effects on Arterial and Venous Intima and Vessel Integrity. J Biol Today's World, 2022, 11(5), 001-004
Received: 01-Sep-2022, Manuscript No. JBTW-22-73381; Editor assigned: 05-Sep-2022, Pre QC No. JBTW-22-73381(PQ); Reviewed: 19-Sep-2022, QC No. JBTW-22-73381; Revised: 29-Sep-2022, Manuscript No. JBTW-22-73381(R); Published: 09-Oct-2022, DOI: 10.35248/2322-3308.11.5.002
Copyright: © 2022 Lichtblau CH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.