Journal of Multiple Sclerosis
ISSN - 2376-0389NLM - 101654564
Research Article - (2018) Volume 5, Issue 1
Background: Previous studies have reported impaired cytotoxic activity in Natural Killer (NK) CD56Dim and CD56Bright cell phenotypes in peripheral blood associated with Multiple Sclerosis (MS). Recently it has been suggested that NK cell phenotype could be associated with new lesions on Magnetic resonance imaging (MRI) and may be used as an immunological indicator of disease activity. This project, for the first time, investigates NK cell cytotoxicity in MS patients with active and stable lesions.
Methods: NK cell cytotoxic activity and NK CD56Dim and CD56Bright cell phenotypes were examined in MS patients using flow cytometry. Isolated NK cells were labelled with antibodies to determine CD56Dim and CD56Bright NK cells and cytotoxic function using target cells (K562). Seven patients (aged 38.0 ± 3.21) with stable Relapsing Remitting MS (RRMS) who had previously received alemtuzumab (Lemtrada®), five patients with active MS (aged 32.66 ± 5.17) on nil medication and five healthy controls (aged 34.4 ± 6.12) participated.
Results: There were no significant differences for NK cell cytotoxic activity and NK CD56Dim and CD56Bright phenotypes between stable RRMS, active RRMS and healthy controls.
Conclusion: Clear associations between NK cell cytotoxicity and clinical MS subtypes in this study were not identified.
Keywords: Natural killer cell function; Natural killer phenotypes; Multiple sclerosis
Multiple sclerosis (MS) is a chronic autoimmune, neuroinflammatory disease of the central nervous system (CNS) [1-4]. MS attacks the myelinated axons in the CNS destroying the myelin and axons to varying degrees causing serious disability [5]. MS has a heterogeneous presentation including sensory and visual disturbances, motor impairments, autonomic dysfunction, fatigue, pain and cognitive deficit [6]. The course of MS is highly varied and unpredictable [5] which correlates with the spatiotemporal dissemination of lesion sites within the CNS [4].
The hypothesis that MS is an autoimmune disease is closely linked to defects in immune regulation [7] and the success of immunosuppressant medications [8]. T-regulatory cells and T-helper cells have been implicated in the development of MS lesions [8,9]. Research suggests that T-regulatory cells become sensitised to myelin and are activated once they enter the CNS through blood vessels [10,11]. B-lymphocytes are believed to produce myelin antibodies through increased immunoglobulin G production in cerebrospinal fluid (CSF) [12]. These antibodies recognise myelin antigens initiating a cascade of events resulting in the formation of acute inflammatory and demyelinating lesions [13,14].
MS is diagnosed using the McDonald criteria which incorporate clinical and laboratory elements to allow early confirmation of the disease to enable earlier decisions regarding disease modifying therapies [15]. MS is clinically diagnosed on the basis of two episodes involving two or more areas of the CNS over time [16]. Additionally, the McDonald criteria incorporate supporting evidence from ancillary tests such as magnetic resonance imaging (MRI) of the brain to demonstrate areas of involvement and also the appearance of new enhancing lesions [17]. The presence of oligo-clonal bands in the CSF contributes to the early diagnosis of MS [12,16,17]. In most patients, the disease is characterised initially by episodes of reversible neurological deficits which are commonly followed by progressive neurological deterioration [5]. MRIs reveal lesions of plaques or scars of the white matter which are caused by immune cell infiltration across the blood-brain barrier (BBB) promoting inflammation, demyelination, gliosis and neuro-axonal degeneration disrupting neuronal signalling [6]. The typical findings of an MRI include juxta-cortical, periventricular, infra-tentorial and spinal cord lesions. Patients may be grouped into four types of MS that are distinguished depending on disease duration and future progression: (1) relapsing-remitting (RRMS); (2) primary progressive (PPMS); (3) secondary progressive (SPMS); and (4) progressive relapsing (PRMS) [9]. This current project focuses on RRMS patients that make up 85% of MS patients. RRMS is defined by symptom relapses or exacerbations followed by periods of remission during which symptoms improve or disappear [5,18].
The cause of MS is unknown, however, it is believed to involve a combination of genetic susceptibility and a non-genetic triggers such as viral, metabolic or environmental factors [5]. Worldwide there are approximately 2.5 million people suffering MS, therefore, posing a significant socioeconomic burden. MS is the most common cause of neurological disability in young adults globally [19], typically presenting in adults 20-45 years of age and occasionally presenting in childhood or late adulthood [5]. The prevalence of MS is much higher in women with ratios as high as 3:1 to men [20].
Currently, there is no single, curative, Food and Drug Administration (FDA)-approved therapy available for MS [5]. The proposed goals of therapy with disease-modifying agents in patients with MS include shortening the duration of acute exacerbations, decreasing their frequency, and providing symptomatic relief. Recent pharmacological investigations suggest that Alemtuzumab (Lemtrada®) results in the depletion of CD56 expressing NK cells, T and B lymphocytes [21]. While this treatment mechanism suggests antibody dependent cellular cytotoxicity (ADCC) in the mechanism of MS [22,23], the treatment does not ultimately prevent progression of MS-dependent disability [24].
The role of innate immunity in the pathogenesis of MS has attracted significant attention with numerous studies focusing primarily on the role of NK cells in RRMS patients [25-31]. NK cells are phenotypically distinguished by the surface expression of CD56 markers and CD16 receptors. Cytotoxic NK cells are CD56Dim and express CD16, whereas CD56Bright NK cells are known as regulatory cells due to the secretion of pro- and anti-inflammatory cytokines [32,33].
The involvement of NK cells in autoimmunity is unknown; however a possible mechanism (e.g., ADCC) may include the rapid release of cytokines modulating interaction between autoreactive T and B lymphocytes [34]. Previous investigations have reported that NK cells are able to lyse axons through the release of proteolytic enzymes, predominantly perforin and granzyme B, and are found in acute inflammatory lesions. In MS, most studies have suggested an impaired cytotoxic activity of NK cells in peripheral blood, which can be associated with disease progression [27,29,30,35-37]. Oger et al. have reported that patients with large asymptomatic MRI lesions had reduced NK cell cytotoxicity [37]. Subsequently, NK cell dysfunction has been implemented in MS pathogenesis and the role of particular NK cell subsets in mediating relapse and remission has been explored [31]. A previous study has shown that decreased CD56Bright NK cells are believed to enable autoreactive T lymphocyte survival contributing to lesion formation [38]. Investigators have suggested a potential therapeutic benefit of NK cells in MS patients as immunomodulatory therapy that increased NK cell numbers decreased auto-reactive T lymphocytes [38]. Additionally, a retrospective MRI investigation reported that CD56Bright NK cells were associated with stable lesion formation [39]. Thereby, there is some evidence for the immunoregulatory role of NK cells in MS.
Although previous research has reported on NK cell phenotypes and cytotoxic activity in MS, investigators are yet to comment on NK cell numbers and activity in MS patients with lesions of different stages. Moreover, these undiscovered mechanisms, may influence both NK cell function and MS disease progression through either common or different pathways at the local environment. This project, for the first time, investigates NK cell cytotoxicity in MS patients and progression of the illness with active and stable lesions.
Study participants
Seven stable MS patients were recruited with no evidence of disease activity after a minimum of 12 months on similar disease modifying medication. Four patients with clinically and radiologically active MS were recruited from specialist neurology outpatient clinics at Gold Coast University Hospital. MS patients were defined in accordance with the 2010 Revised McDonald diagnostic criteria for MS. Four MS patients were originally diagnosed with Clinically Isolated Syndrome (CIS) before being diagnosed with new and active lesions. Five healthy controls (HC) who reported no history of autoimmune disease, thyroid disease, cardiovascular disease or diabetes were recruited using a participant database at the National Centre for Neuroimmunology and Emerging Diseases (NCNED). This study was approved by the Griffith University Human Research Ethics Committee (MSC/18/13/ HRE). Written consent was provided by each participant prior to blood collection.
Peripheral blood mononuclear cell isolation and natural killer cell isolation
Participants donated 45 ml of whole blood collected in ethylendiaminetetraacetic acid (EDTA) tubes. Routine full blood analysis was performed on 5 ml of blood within 4 h of collection. Participants were screened and excluded if blood parameters were abnormal.
Peripheral blood mononuclear cells (PBMCs) were isolated from 40 ml of whole blood by centrifugation over Ficoll-Paque density gradient medium (Ficoll-Paque Premium; GE Healthcare, Uppsala, Sweden) as described previously [40]. PBMCs were stained with trypan blue (Invitrogen, Carlsbad, CA) to determine cell count and cell viability, and adjusted to a final concentration of 5 × 107 cells/ml.
NK cells were isolated from PBMCs using immunomagnetic negative selection (EasySep Negative Human NK Cell Isolation Kit; Stem Cell Technologies, Vancouver, BC, Canada) as previously described by Huth et al. [41].
NK cell purity and phenotype
NK cell purity was measured following 20 min incubation with CD56 Pe-Cy7 (0.25 μg/5 μl) and CD3 APC (0.25 μg/5 μl) antibodies (BD Bioscience, San Jose, CA, USA) at room temperature in the dark. Flow cytometric analysis using a LSR-Fortessa X20 (Becton Dickinson [BD] Biosciences, San Diego, CA, USA) determined percentage of CD56+CD3- cells by recording 10,000 events.
NK cell immune phenotyping was performed on purified NK cells using CD16 BV650 (0.25 μg/5 μl) and CD56 Pe-Cy7 (0.25 μg/5 μl) antibodies (BD Bioscience, San Jose, CA, USA) incubated for 20 min at room temperature in the dark. Flow cytometric analysis recording 10,000 events was used to determine the percentage of CD56Bright and CD56Dim NK cell populations.
K562 cell line was cultured in RPMI-1640 (Invitrogen Life Technologies, Carlsbad, CA, USA) supplemented with 10% foetal bovine serum (FBS) (Invitrogen Life Technologies, Carlsbad, CA, USA). K562 cells were passaged every 48 h and incubated at 37oC with 5% CO2. K562 cells were employed as target cells for the NK cell cytotoxicity assay.
NK cell cytotoxicity
Following isolation, the concentration of NK cells was adjusted to 5 × 105 cells/ml. NK cells were labelled with Paul Karl Horan (PKH-26) (3.5 μl/test) (Sigma-Aldrich, St. Louis. MO, USA) and incubated with K562 cells for 4 h at 37°C with 5% CO2 in RPMI-1640 supplemented with 10% FBS. NK cell cytotoxicity was analysed at various effectortarget (E:T) ratios including 12.5:1 and 6.25:1. Following incubation, cells were stained using Annexin V (2.5 μl/test) (BD Bioscience, San Jose, CA, USA) and 7-amino-actinomycin (7-AAD) (2.5 μl/test) (BD Bioscience, San Jose, CA, USA) to determine apoptosis of K562 target cells using flow cytometry recording 10,000 events [42]. The percentage of NK cell lytic ability was calculated from the below equation:
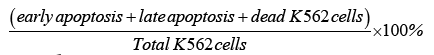
Data analysis
Data was exported from FACSDiva version 8.1 (BD Biosciences, San Diego, CA, USA). Statistical analysis was performed using SPSS version 24 (IBM Corp, Version 24, Armonk, NY, USA) and GraphPad Prism version 7 (GraphPad Software Inc., Version 7, La Jolla, CA, USA). All data sets were tested for normality using the Shapiro-Wilk test. Data between active MS, stable MS and HC participants were compared using the one-way ANOVA test. Significance was set at p<0.05. Data is presented as mean ± standard error of mean (SEM) unless otherwise stated.
Patient characteristics, blood parameters and NK cell purity
A total of 17 participants were included in this study; five HC, five active MS and seven stable MS patients. The epidemiological characteristics of all participants and routine full blood analysis are summarised in Table 1. There was no significant difference in age and gender between the three groups.
| Characteristic | Active | Stable | HC |
|---|---|---|---|
| Gender, n (%) | |||
| Female | 3 (60%) | 4 (57%) | 3 (60%) |
| Male | 2 (40%) | 3 (43%) | 2 (40%) |
| Age | 32.66 ± 5.17 | 38.0 ± 3.21 | 34.40 ± 6.12 |
| Pathology result | |||
| HB | 140.2 ± 3.25 | 1393.42 | 135 ± 6.88 |
| WCC | 5.50 ± 0.76 | 5.61 ± 0.75 | 5.64 ± 0.38 |
| Plt | 203.83 ± 52.14 | 262.71 ± 13.12 | 263 ± 35.53 |
| Lymphocyte | 1.54 ± 0.55 | 1.68 ± 0.89 | 1.67 ± 0.12 |
| Monocyte | 0.23 ± 0.04 | 0.32 ±.048 | 0.27 ± 0.052 |
| Neutrophil | 3.82 ± 1.12 | 4.04 ± 0.72 | 3.57 ± 0.45 |
| Basophils | 0.02 ± 0.004 | 0.02±.0036 | 0.22 ± 0.007 |
| Eosinophils | 0.08 ± 0.03 | 0.16 ± 0.43 | 0.08 ± 0.03 |
| Patients’ demographic features and pathology parameters measured in multiple sclerosis subgroups and healthy control groups. Comparisons of blood parameters between the MS subgroups and HCevealed no significant differences. No significant differences were observed in patient age distribution. Data presented as mean ± standard deviation unless otherwise stated. Abbreviations: MS: Multiple Sclerosis; HC: Healthy Control; Hb: Haemoglobin; WCC: White Cell Count;, Plt: Platelet; SEM: Standard Error Of Mean | |||
Table 1: Epidemiologic characteristic of patients and pathology results.
Stable MS patients consisted of seven patients diagnosed with RRMS based on McDonald criteria. They had at least one course of Alemtuzumab (Lemtrada®) infusions more than six months prior to commencing the current study. These patients had also been clinically and radiologically stable for at least six months. The mean age of patients with stable MS was 38.0 ± 3.21 years and 62% of them were females.
Five patients with new radiological and clinical relapses within three months of the study were included in the current investigation. Four patients had previously been diagnosed with CIS and were on high doses of vitamin D. The vitamin D level for each patient was around 80-100 ng/ml. One patient with active MS was diagnosed eight months prior to the current study; however she refused any medication for MS and suffered relapses of MS six weeks before the study. She did not receive any corticosteroid or any other medication known for interfering with NK cells subset number or function. The age of patients with active lesions was 36.22 ± 5.17 years of which one patient was male.
Five HC reported having no autoimmune, cardiovascular or thyroid diseases and were not currently pregnant or breastfeeding. Of the five participants three were female and the mean age was 34.4 ± 6.12.
There were no significant differences reported for full blood analysis. All parameters were within normal range for gender and age (Table 1).
NK cell purity was performed using flow cytometry following NK cell isolation using commercial kits. NK cell purity for active MS patients was 98.96% ± 0.56, stable MS patients was 96.08% ± 3.38 and HC was 99.66% ± 0.30 (Figure 1).
NK cell phenotype
NK cell phenotype was determined using flow cytometry by assessing CD16 and CD56 expression for the three groups: HC (n=5), stable MS patients (n=7) and active MS patients (n=5). There was no significant difference in CD56Dim NK cells in HC compared with stable and active MS, nor was there any difference in CD56Dim NK cells in HC compared with MS patients and between stable and active MS subgroups (Figure 2). Moreover, no significant difference was reported in CD56Bright NK cells between HC and MS patients or CD56Bright NK cells between stable and active MS patients.
NK cell cytotoxicity
Cytotoxic activity was determined using flow cytometry to assess NK cell lysis of the tumour target K562 cell line for the three groups: HC (n=5), stable MS patients (n=7) and active MS patients (n=5). There was no significant difference in NK cell cytotoxicity in HC compared to stable and active MS patients at 12.5:1 effector-target (E:T) ratio (Figure 3A) or between HC, stable and active MS patients at 6.25:1 E:T ratio (Figure 3B). Additionally, no significant difference in NK cell cytotoxicity was observed between stable and active MS subgroups.
Figure 3: (A) Natural Killer cell Cytotoxic Activity at 12.5:1 E:T ratio. Bar graphs representing NK cell cytotoxic activity at 12.5:1 ratio in three groups of participants: patients with stable MS, active MS and HC. (B) Natural Killer cell Cytotoxic Activity at 6.25:1 E:T ratio. Bar graphs representing NK cell cytotoxic activity at 6.25:1E:T ratio in three groups of participants: patients with stable MS, active MS and HC.
This current pilot investigation has, for the first time, assessed NK cytotoxicity and phenotypes in MS patients with differing clinical presentation. This current investigation using isolated NK cells through flow cytometric methods identified no significant difference between NK cell cytotoxicity or NK cell phenotypes between HC and MS patients who were clinically diagnosed for stable or active lesions as determined by MRI.
Our research findings are consistent with previous investigations in untreated MS patients or patients with CIS suggestive of MS when compared to HC [43]. Rationale for this outcome may be due to NK cell phenotypes being CNS specific and not blood specific tropism of NK cell phenotypes [44,45]. Previous investigators have reported the majority of intrathecal NK cells in healthy individuals, MS patients and patients with other neurological diseases are CD56Bright NK cells [46-48] are not MS specific, but rather CNS specific. Thus NK phenotypic changes may reflect organ-specific, rather than blood-specific, where lymphatic vessels in the brain [45,49] may be the route of entry for CD56Bright NK cells [50]. Moreover, previous investigators have reported CD56Bright NK numbers in untreated MS patients or patients with clinically isolated syndrome suggestive of MS [51] was similar in MS patients and HC. However, upon stimulus with pro-inflammatory cytokines, CD56Bright NK cells from MS patients suppressed the proliferation of autologous CD4+ T cells compared with those from HC. Hence the role of cytokines in the organ specific microenvironment and different NK cell phenotypes occurring with varying clinical presentation of MS may be important.
The immunoregulatory function of NK cells and cell surface expression of activating and inhibitory receptors for NK phenotypes may play a critical role in MS disease progression. NK cell function is regulated by balancing the activating and inhibitory signals provided by the respective receptors, which have been shown to be differentially expressed by NK cell subsets [52]. The activating natural cytotoxicity receptors NKp30, NKp44 and NKp46 varies in both populations as well as various soluble factors modulate the functional capabilities and development of NK phenotypes [53] in particular, IL-2, IL-12 and IL-15 which affect NK cell biology as well as IL-21, a cytokine with pleiotropic effects on various cell types [54]. The corresponding IL-21 receptor (R) is expressed on B cells, T cells, dendritic cells and equally on CD56Dim and CD56Bright NK cells [55]. Signalling of IL-21R is mediated via CD132, which is also a component of IL-2R, IL-7R, and IL-15R [56]. A recent study demonstrated the development of CD56Dim NK cells from CD34+ haematopoietic stem cells in the presence of FMS-like tyrosine kinase 3 ligand (flt3L), stem cell factor, IL-15 and IL-21. In contrast, without IL-21, the generation of CD56Bright NK cells lacking CD16 and Killercell immunoglobulin-like receptors (KIRs) is favoured [57]. Moreover, a previous investigation reported peripheral NK cell phenotypes in MS patients in remission had increased IL-5 expression of CD95 [58], which inhibited the production of Interferon-γ (IFN-γ) [59]. These researchers also reported a further subset of these MS patients had CD11c-high (not producing IL-5) and CD11c-low (producing IL-5) subsets where those MS patients identified CD11c-high patients are at a higher risk of relapse [60].
Previous exposure to pathogens and infection may also influence the development of MS [11] as it has been suggested to influence NK phenotypes and NK cell functions [61-63]. Martinez-Rodriguez et al. investigated the expression of NKG2C receptor on NK cells from MS patients and controls, in relation to their Cytomegalovirus positive (CMV+) serostatus and to the NKG2C genotype. They reported expansion of NKG2C+ NK cells in CMV patients was associated to lower risk of disease progression. Differently from CMV, infection with Epstein-Barr virus, which has also been associated with an increased risk of MS, has also been reported to increase NKG2A+ CD56DimNK cells [64,65]. Hence previous exposure may provide a possible rationale for the phenotype findings reported in this investigation.
A number of studies have reported a significant reduction in NK cytotoxicity using peripheral blood mononuclear cells [27,29,30,35-37], compared to with this pilot investigation of isolated NK cells. Moreover, these previous studies employed in vitro chromium51 release assay using K562 tumour cells as the target and is not without limitations [66]. Importantly, through NK cytotoxic activity, and through the production of type 2 cytokines such as IL-5 and IL-13, NK cells may lyse and suppress T helper 1 autoimmune cells which mediate the inflammatory process in the CNS of patients with MS [58,67,68]. Activation of NK cell effector function is stringently regulated by surface receptors and the significant increase in KIR2DL5 on CD56BrightCD16−/Dim NK cells and CD94 on CD56DimCD16+. The KIR2DL5 receptor relays inhibitory signals, and increased expression of the KIRs on peripheral NK cells has been associated with decreased cytotoxic activity and production of IFN- γ [69,70]. The cytoplasmic tails of the inhibitory receptors following receptor ligation, suppress NK cytotoxic activity and cytokine production [71,72]. CD94 is a homodimer structure which associates with NKG2- A, B, C, E or H to form heterodimers capable of regulating NK activity through the transmission of activating or inhibiting signals [73,74]. CD94 is known as a regulatory molecule and alterations in the expression of CD94 on CD56DimCD16+ NK cells from the MS cohort may affect the ability of NK cells to induce cytotoxic lysis of target cells due to changes in signalling thresholds [73]. As the regulation of NK cell activity is governed by both activating and inhibitory signals, the significant increases in KIR2DL5 and CD94 surface expression may contribute to threshold levels [73] for cytotoxic function in MS cohorts. In particular, increased inhibitory signals from KIR2DL5 may set a higher activation threshold, masking important activating signals for NK effector function in patients with MS [72,75]. Inhibition of NK cell activity through enhanced expression of specific KIRs on NK cells from patients with MS has been associated with an increased susceptibility to infections from the herpes virus [69,70]. The expression of these cell surface receptors may, in part, explain differences in NK cell phenotype and function.
In addition, a longitudinal investigation of NK cytotoxicity in RRMS patients has reported “valleys” in NK killing activity that last 4-5 weeks, where transient deficits in cytolytic activity may provide a rationale for the varied results for NK cell function through the death receptor and cytolytic pathways. Importantly, these researchers found a significant correlation between valleys in NK cell killing activity and new or enlarging lesions on MRI. These authors concluded that NK cell killing activity represent periods of susceptibility for the development of active lesions on MRI and NK valleys are the result of cells with an NK phenotype being unable to deliver a “lethal” hit to targets [35]. In this pilot investigation due to low subject numbers these effects may not have been able to be reported.
Finally, in a cross sectional study of untreated MS patients researchers identified a relative deficiency of CD16+/Perforin+ NK cells relative to HC [76]. Low levels of CD16+/Perforin+ lymphocytes may provide an explanation for cytotoxic changes in MS patients, however, due to the low sample number in the present study, this was not able to be observed.
This pilot study is the first to investigate NK cell cytotoxicity and phenotypes using isolated NK cells between HC and MS patients who were clinically diagnosed for stable or active lesions as determined by MRI. Our research findings are consistent with those of previous investigators showing no significant differences in NK cell phenotype or function in untreated MS patients or patients with clinically isolated syndrome suggestive of MS compared with HC, however, further investigations are warranted to assess cytokine and cell surface expression.
The authors have reviewed and approved the final version of this manuscript and declare no conflict of interest in the research presented.
This research was supported by funding from the Stafford Fox Medical Research Foundation, Mr Douglas Stutt, Blake Beckett Foundation, Alison Hunter Memorial Foundation and the Queensland Government.
Data sharing is not applicable to this article as no datasets were generated under the Griffith University Intellectual Property policy. Supporting data for this study is included within the article.