Mini Review - (2021) Volume 7, Issue 1
Over the past 10 years technology has enabled members of the scientific community to learn more about the structures associated with neurodegenerative diseases on a microscopic level. By looking at how proteins such as tau and amyloid-beta (Aβ) contribute to the pathogenesis of neurodegenerative conditions such as Alzheimer’s Disease (AD), we increase the probability of manufacturing drugs that are better able to control symptoms and offer a possible cure. This essay will explore how the scientific community diligently works on bringing us one step closer to answering the plethora of questions surrounding what affects the universe we carry on our shoulders.
AD is a chronic neurodegenerative condition that mainly associated with memory impairment and affects 6% of those above the age of 65 who go onto to develop speech disorders, psychological and psychiatric changes which affect their quality of life [1]. Hypothesis to explain the pathogenesis of AD include the cholinergic hypothesis Amyloid Osaka mutation genetics and the Tau hypothesis [2-4]. Tau hypothesis states that the hyper phosphorylation of Tau, which forms Neurofibrillary Tangles (NFTs) causes neuronal cell death while the amyloid hypothesis states that AD is primarily caused by the deposition of oligomeric or fibrillary amyloid β peptides in the brain [5,6]. For many years the scientific community used the amyloid hypothesis to explain the pathology behind AD, which led to the development of drugs targeting the reduction of Aβ formation. These drugs have thus far failed at showing any form of symptomatic relief.
The formation of Aβ in small quantities is a normal physiological phenomenon. It only becomes pathological when an excessive amount of Aβ is deposited in the brain due to an imbalance in the degradation and formation of Aβ [7]. Mutations in the genes encoding for Amyloid Precursor Protein (APP) and Presenilins (PS) contribute to the onset of AD. APP is a membrane bound protein found in cell membranes and is involved in synaptic formation and repair [8]. Parts of the APP molecule are cleaved by enzymes known as secretases (α, β and γ-secretase) which produce many by-products, one of which is Aβ.
Mutations in PS cause the γ-secretase enzyme to defunction. This mutation reduced the production of certain forms of Aβ (Aβ40), while increasing the formation of others (Aβ42), which caused an overall increase in Aβ deposition [9]. Furthermore, ineffective function of γ-secretase meant that compounds cleaved from APP weren’t being removed by conversion to other compounds effectively which contributed to the neurotoxicity leading to neuronal cell death and synaptic damage (Figure 1) [10].
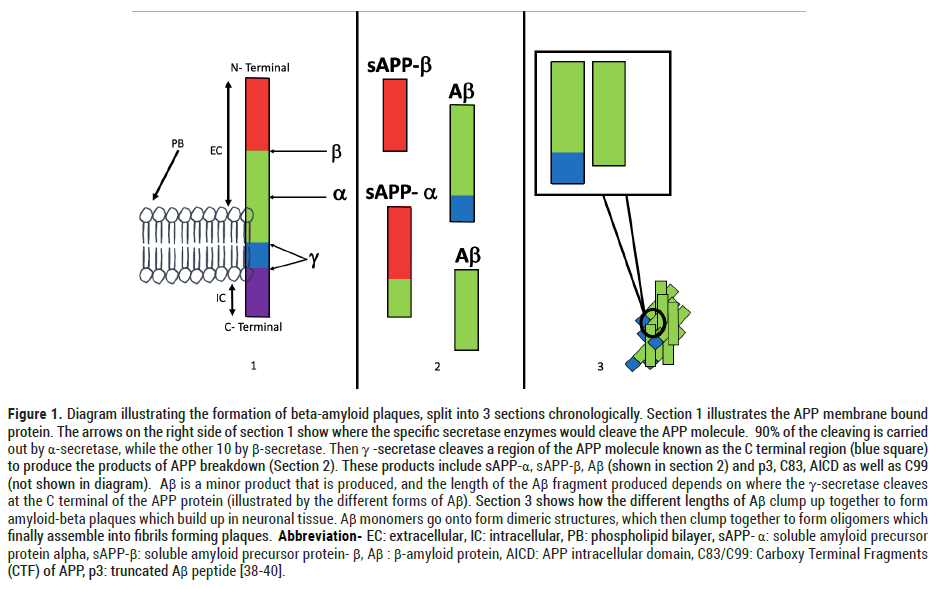
Figure 1: Diagram illustrating the formation of beta-amyloid plaques, split into 3 sections chronologically. Section 1 illustrates the APP membrane bound protein. The arrows on the right side of section 1 show where the specific secretase enzymes would cleave the APP molecule. 90% of the cleaving is carried out by α-secretase, while the other 10 by β-secretase. Then γ -secretase cleaves a region of the APP molecule known as the C terminal region (blue square) to produce the products of APP breakdown (Section 2). These products include sAPP-α, sAPP-β, Aβ (shown in section 2) and p3, C83, AICD as well as C99 (not shown in diagram). Aβ is a minor product that is produced, and the length of the Aβ fragment produced depends on where the γ-secretase cleaves at the C terminal of the APP protein (illustrated by the different forms of Aβ). Section 3 shows how the different lengths of Aβ clump up together to form amyloid-beta plaques which build up in neuronal tissue. Aβ monomers go onto form dimeric structures, which then clump together to form oligomers which finally assemble into fibrils forming plaques. Abbreviation- EC: extracellular, IC: intracellular, PB: phospholipid bilayer, sAPP- α: soluble amyloid precursor protein alpha, sAPP-β: soluble amyloid precursor protein- β, Aβ: β-amyloid protein, AICD: APP intracellular domain, C83/C99: Carboxy Terminal Fragments (CTF) of APP, p3: truncated Aβ peptide [38-40].
In 1984, an article published by Glenner and Wong about the ‘Alzheimer’s Disease gene’ on chromosome 21 containing the amino acid sequence for the formation of amyloid beta (Aβ) protein deposits set up the foundations of the amyloid hypothesis. The amyloid hypothesis states that the accumulation of Aβ due to the imbalance in its degradation and production leads to the formation of NFTs and neuronal cell death in patients with AD [11]. Treatments to target the accumulation of Aβ were developed, none of which were successful [12]. Since 2010 ample evidence has been produced to prove that the accumulation of Aβ deposits themselves may not be contributing to the cognitive decline that is described in past medical literature, alluding to the involvement of other proteins. In 2013, a study looking amyloid protein deposition and cognitive decline in model AD mice, showed no correlation between the two [13-14].
The approval for the use of Positive Electron Tomography (PET) amyloid ligands in the investigation of AD, such as the 18F-florbetapir in 2012 allowed scientists to gain a visual image of the deposition of Aβ plaques in neuronal tissue [15]. In 2017, scientists were able to visualize the atomic structure Tau tangles found in an Alzheimer’s patient using cryo-electron microscopy (Cryo-M) technology which has since helped us in imaging studies of the brain [16].
A spanner was thrown in the works early on when studies such as those published by Chetalat et al. in 2013 showed that there was no statistically significant link between Aβ deposits and symptoms such as cognitive decline associated with AD. The individuals who were asymptomatic had a similar number of Aβ deposits to those who had symptomatic AD [17]. Despite these recent findings, parts of the theory amyloid hypothesis still may stand relevant in the pathogenesis of familial AD (fAD). The process by which Aβ deposits are produced, involves improper metabolism of the products of degradation of APP; a membrane bound protein (Figure 1). As the products of metabolism of APP are removed from the neuronal tissue at a fast rate, we could determine that accumulation of degradation products of APP, fragments involving the APP-C terminal carry an element of neurotoxicity [18,19]. Furthermore, a paper published by Woodruff et al. in 2016 detailed how the accumulation of C-terminal fragments of APP disrupted endocytic trafficking within and between axons [20].
This further provided evidence showing that Aβ deposits that weren’t contributing to neuronal dysfunction but the incorrect metabolism of APP and accumulation of its C-terminal fragments. Hence it was postulated that the pathogenesis of fAD began due to improper metabolization of APP in the brain. Scientists are still debating on whether this explanation would answer the mysteries surrounding sporadic AD as well [21]. When analyzing what other sorts of pathologies could contribute the pathogenesis of AD, attention was brought to the Tau Hypothesis.
Human Tau protein is found in the axons in the brain and is involved in modulating microtubules formed by tubulin found in axons. It’s interaction with tubulin helps regulate neuronal polarity, axon outgrowth and transport within the axon [22]. In neurodegenerative conditions such as Alzheimer’s disease (AD), Tau undergoes post translational hyperphosphorylation which causes it to form Neurofibrillary Tangles (NFTs) in neuronal bodies (known as neurofibrillary threads if they are found in the axons or dendrites of neurons). These tangles then dissociate from the microtubules and form insoluble aggregates [23,24]. Full length Tau then assembles into filaments, which in pathological conditions spread throughout the brain where the shorter filaments have a higher degree of spreading than longer filaments [25].
Alternative splicing of the tau gene found on chromosome 17 allows six isoforms of Tau to form (Figure 2). In summary, tau protein isoforms can be classified into 4R isoforms and 3R isoforms, where the 4R isoforms consists of exon 10, while the 3R isoforms do not. These aggregates form filamentous inclusions which then spread throughout the neural network of the brain [26].
Figure 2: Table summarizing the different sort of exons in each of the six isoforms of Tau. X: Exon Present, Exon absent [41].
Tau hypothesis states that AD is predominantly caused by the accumulation of Tau.
Until recent years, the role of Tau pathology in the development of AD was not studied extensively (Table 1).
| Name | Mode of Action |
|---|---|
| Bapineuzumab | Target Amyloid-beta plaques |
| Solanezumab | Targets monomeric amyloid-beta deposits * |
| Crenezumab | Engineered to clear excess amyloid-beta deposits * * |
| Gantenerumab | Microglial-include phagocytosis of the amyloid-beta deposits |
Abbreviations: FAD: Familial Alzheimer’s Disease; APP: Amyloid Precursor Protein; AD: Alzheimer’s Disease [42-45].
Note: *targets the amyloid-beta compound at an earlier stage than Bapineuzumab. **did this while trying to negate the negative effects of previous treatments such as vasogenic oedema. ***However higher doses have seemed to reduce the number of amyloid deposits (trials will continue till 2024).
Table 1. A timeline summarizing how the understanding of the pathology behind Alzheimer’s has changed over the years.
A paper published by Takashi et al. in 2015 talks about how APP binds the tau thereby allowing for its accumulation in the brain in patients with AD, not Aβ as previously believed. They also noticed that (seed dependent) tau aggregation did not occur in cells that lack the extracellular component of APP, while the incorporation of Aβ 42 did not enhance tau formation or deposition in the cell either [27]. They concluded that although Aβ was thought to be important in the propagation of Tau AD brains, it may not have been as significant to the pathogenesis as much as we thought it did. Such results along with the disappointing performance of therapies targeting Aβ plaques led the development of therapies targeting the accumulation of Tau in the brain.
One of the first pathological processes associated with AD is the phosphorylation of Tau [28]. Enzymes known as kinases that phosphorylate Tau were upregulated in those who had AD while phosphatases were down regulated [29]. Phosphatases are enzymes involved in the removal of phosphate from a molecule, and in the brain Protein Phosphatase 2 A (PP2 A) is involved in around 70% of the Tau Phosphatase activity [30,31]. Memantine, a PP2 An activator was used in the treatments of AD early on. Memantine underwent many clinical trials starting in 2004 up until 2018. Although Memantine showed small improvement in cognitive function, a study carried out in 2018 showed that it didn’t have a substantial effect in reducing AD symptoms compared to a placebo [32]. Methylene Blue (MB) was believed to reduce Tau aggregation in the brain by inhibiting a process known as heparin induced Tau filament formation [33]. However, clinical trials that began in 2007 which carried on till 2020 did not provide concrete evidence as to whether it produced clinically significant results. Phase II and III clinical trials were deemed inconclusive due to improper technique while certain results showed that MB may not even be binding to the cells as described before due to color changes seen in urine. Furthermore, the study of the effect of MB on Tau in Zebrafish in 2010 showed that it had no effect on Tau induced polyglutamine toxicity [34]. Such failures in therapies surrounding Tau targets have caused attention to be shifted to immunotherapies targeting Tau (Table 2 and Figure 3).
| Isoform | Exon 2 | Exon 3 | Exon 10 |
|---|---|---|---|
| 0N3R | - | - | - |
| 1N3R | x | - | - |
| 2N3R | x | x | - |
| 0N4R | - | - | x |
| 1N4R | x | - | x |
| 2N4R | x | x | x |
Table 2. List of monoclonal antibodies that were mainly used to target Aβ deposition which had failed to provide evidence that the drug made a significant difference in curbing AD [38-49].
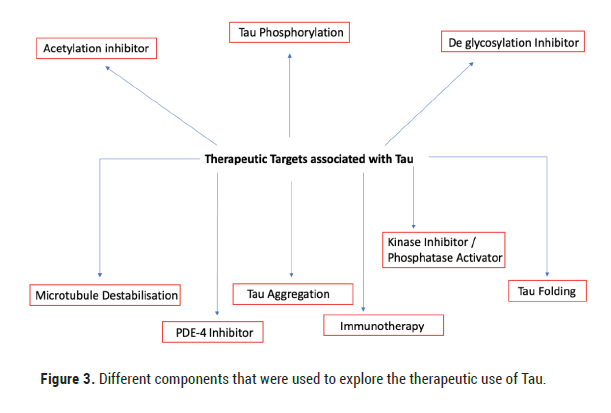
Figure 3: Different components that were used to explore the therapeutic use of Tau.
Bapinezumab, one of the first monoclonal antibodies that targeted the amyloid-beta plaques showed a reduction in the Tau protein concentration in CSF not significant symptomatic improvements of AD (2016). Its side effects included vasogenic edema, which further limited its potential therapeutic [35]. This prompted scientists to develop safer antibodies such as Solanezumab. Although less likely to cause vasogenic edema, it did not reduce the Tau protein concentration in CSF or affect distribution of amyloid fibrils in the brain (2014). There was also no improvement in cognitive and functional symptoms associated with AD [36]. These provide a few examples of Tau therapies that have failed due to adverse side effects or lack of symptomatic improvement in AD. Despite what we’ve learnt over the years regarding Tau’s involvement in AD, Aducanumab; an antibody that binds aggregated collections of Aβ showed promising results. Patients receiving the highest dose of Aducanumab showed a significant decline in cognitive and functional symptoms associated with AD [37].
Although much advancement have been made in the medical community with the help of technology in understanding Tau and its pathogenic effects, there is still a long way to go. Targeting different targets associated with Tau proteins as well as amyloid-beta, while developing drugs that don’t carry too many side effects will prove to be the most effective way of dealing with the manner. With the new interest shown in Tau immunology and targeted PET scanning, one could only hope that in the next 10 years, we will be closer to finding effective medication against AD and other pathologies.
Citation: Peris R. Alzheimer’s Disease and its Treatments: A Timeline of Events. J Neurosci Neuropharm, 2021, 7(1), 001-004.
Received: 20-May-2021 Published: 10-Jun-2021
Copyright: © 2021 Peris R. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.