Short Communication - (2021) Volume 6, Issue 6
Primary hyperthyroidism is the result of overproduction of thyroid hormone resulting in the classic symptoms of tachycardia, weight loss, diaphoresis, and hyper defecation. There are multiple common causes to include Graves’ disease, toxic multinodular goitre, and solitary toxic adenomas. Marine Lenhart Syndrome (MLS) is a rare cause of hyperthyroidism, caused by a coexistence of constitutively active thyroid nodules and Graves’ disease. In the original document of Marine and Lenhart, there is no distinction made between the autoimmune phenomenon of Graves’ disease and the solitary toxic nodule of Plummer’s disease. Rather they are both considered to be the manifestation of the same disease. However, in the current era of radionuclide technology, a clear distinction of MLS can be seen with diffuse uptake in the thyroid gland and focused enhancement in the toxic nodules. Therefore what was previously described as one entity is now distinct as Graves’ disease and Plummer’s disease. It is also becoming increasingly clear within the literature that there is also a new phenomenon of post-radioiodine immunogenic hyperthyroidism in patients with toxic nodules and elevated auto antibodies. Therefore in order to properly treat and manage patients, a new definition of MLS may need to be proposed.
Marine Lenhart Syndrome (MLS) • Hyperthyroidism • Radioiodine therapy • Autoantibodies • Graves’ disease
The term Marine Lenhart syndrome (MLS) has been assigned to this presentation with the coexistence of Graves’ disease and toxic adenomas and has become more frequently recognized within the literature. However the text by Marine in 1940 failed to appropriately distinguish these two conditions noting toxic adenoma and immunogenic goitre as one condition [1]. The syndrome as described in 1972 by David Charkas based off the original observations of Marine and Lenhart was defined as patients with Graves’ disease and toxic adenomas that exhibit increased radioiodine uptake with TSH stimulation, appear hypo functioning in relation to extra nodular tissue on RAIU and scan, are poorly responsive to RAI ablation, and exhibit increased radioiodine uptake after RAI ablation therapy [2,3]. Thus, one of the key distinctions of the toxic adenomas of MLS compared to those autonomous nodules found in Plummer’s disease was their TSH dependence. A recent literature review in 2011 by Bier sack suggested that MLS may be a conjoined syndrome; the classical Graves’ disease/toxic adenoma vs Graves’ disease post RAI ablation with autonomously functioning thyroid nodules. Examination of the previous definition of toxic adenomas by Plummer defines hyperthyroidism as a disease caused by two separate entities (Toxic adenoma and exophthalmic goitre) [1]. Thus the original term may not apply to what we now know to be two separate diseases and the medical literature needs to be updated to reflect the origin of the joint presentation.
The prevalence of this syndrome has been reported to be between 2.7%-4.1% of hyperthyroidism diagnoses, though is likely an under recognized cause of hyperthyroidism [2]. Over the last decade there has been multiple case reports published within the literature of the manifestation of autoimmune induced hyperthyroidism following radioiodine therapy in patients with solitary toxic adenomas [1]. Primary hyperthyroidism is most commonly due to Graves’ disease, accounting for 60%-80% of hyperthyroid cases [1,2]. Graves’ disease is characterized by serum antibodies against TSH receptors in correspondence with T cell mediated immunity. Other causes such as Toxic adenomas or toxic multinodular goitres, also known as Plummer’s disease, are a result of several somatic point mutations in the third trans-membrane loop of the TSH receptor leading to autonomous function in absence of TSH [3]. The presence of thyroid nodules coincides with Graves’ disease in 25%- 30% of patients [2]. Though both disease processes result in thyrotoxicosis, their mechanism of actions are distinct (Figures 1-3) indicates a classical diagnostic image of MLS on both US and RAIU). The incidence is especially high in those patients with pre-existing autoantibodies which is not unusual [2]. Due to the clonal origin of toxic nodules, activating thyrotropin receptor mutations can be present and amplify the activity of the nodule which leads to increased risk of “post-radioiodine immunogenic hyperthyroidism” [3,4]. Hence there remains controversy regarding formal diagnosis due to variations in imaging techniques, presentation, and onset [5,6]. Thus the definition and diagnostic criteria for Marine Len hart Syndrome have subsequently undergone multiple revisions.
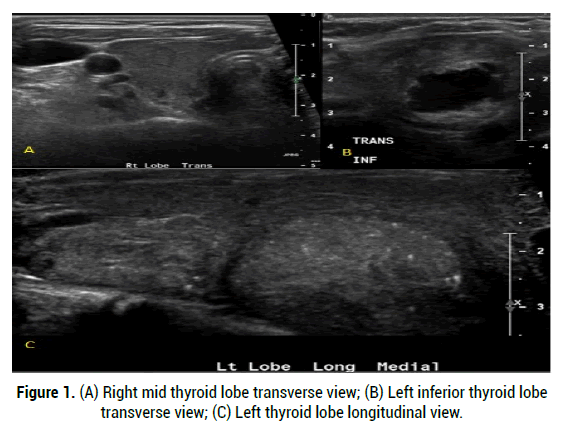
Figure 1: (A) Right mid thyroid lobe transverse view; (B) Left inferior thyroid lobe transverse view; (C) Left thyroid lobe longitudinal view.
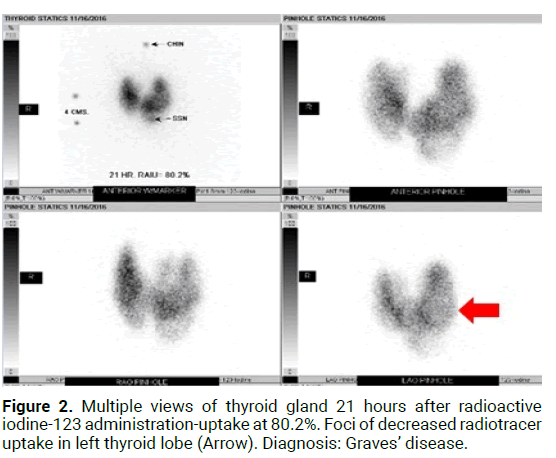
Figure 2: Multiple views of thyroid gland 21 hours after radioactive iodine-123 administration-uptake at 80.2%. Foci of decreased radiotracer uptake in left thyroid lobe (Arrow). Diagnosis: Graves’ disease.
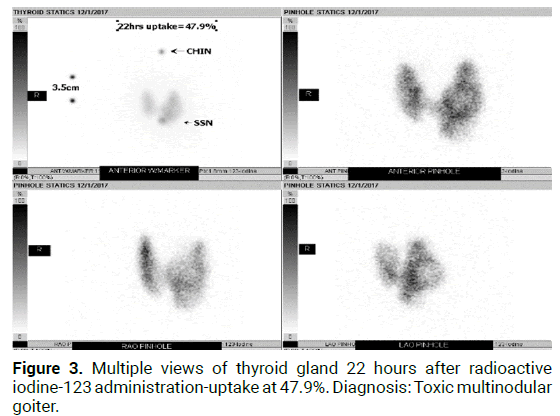
Figure 3: Multiple views of thyroid gland 22 hours after radioactive iodine-123 administration-uptake at 47.9%. Diagnosis: Toxic multinodular goiter.
Recently documented cases of MLS include cases of coexisting Graves’ disease and hyper functioning nodules at the time of diagnosis, cases of Graves’ disease with “cold” nodules at the time of diagnosis that then later were confirmed as hyper functioning, and cases of development of toxic adenomas years after successful treatment of Graves’ disease [7-13]. This highlights the need for a unified definition of MLS, which was proposed by Neumann, et al. [8]. These proposed diagnostic criteria included confirmed hyperthyroidism based on thyroid function tests with positive thyroid autoantibodies consistent with Graves’ disease, RAIU scan revealing hyper functioning nodules that correspond to nodules seen on ultrasound on a background of diffusely increased radioiodine uptake, and confirmation of the presence of a follicular adenoma or hyperplastic lesion on pathologic analysis [8]. Pathologic analysis is important as there have been cases reported in the literature thus far of MLS patients with the additional finding of papillary thyroid carcinoma identified in an associated nodule with the most recent being published by Mehmet, et al. of a unique case of papillary thyroid carcinoma in a hyper functioning nodule [14]. This definition also encouraged a transition from using the term “autonomous” nodules to describing them as functional nodules to include the subset of MLS cases with apparent TSH dependence of the associated nodules. In fact, prior proposed definitions included the requirement for the associated nodules to demonstrate TSH dependence [15]. Damle, et al. note that the amount of radioiodine required to relieve the hyperthyroidism in these patients is greater than the corresponding mean, median or mode dose for diffuse toxic goitre thought to be a result of the large size as well as the radio-resistance of the goitres in this disease [16].
Marine Lenhart Syndrome remains a rare and poorly defined entity that should be considered different both diagnostically and therapeutically from any ethology of thyrotoxicosis in isolation. A broader definition and increased awareness is needed to allow for better understanding and improved treatment guidelines for this syndrome.
Citation: Essien F. Does Marine Lenhart Syndrome Really Exist? Med Rep Case Stud, 2021, 06(6), 226
Received: 15-Oct-2021 Published: 05-Nov-2021, DOI: 10.35248/2572-5130.21.6.226
Copyright: © 2021 Essien F. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : NO