Research - (2024) Volume 15, Issue 2
Background: Decreased AQP 4 expression causes decreased circulatory function of the glymphatic system, and abnormal deposition of proteins such as A β 42 and Tau in the brain. The loss of AQP 4 may be a risk factor for Alzheimer's disease.
Objective: To explore whether TGN-020 (AQP 4 blocker) damages learning and memory, aggravates A β deposition, and whether the combination of DORA and TGN rescues learning and memory impairment in mice.
Methods: During the 28-day intervention period, APP/PS1 mice (AD model mice) received drugs by intraperitoneal injection daily at the start of the light phase (6:00 am). Mouse learning and memory were assessed by the Y and Morris water mazes. After analysis of behavioral parameters, Aβ deposition in the mouse brain was evaluated by immunohistochemistry.
Results: 30 mice (2 died, one in each of TGN + 30 and control groups, mortality of 6.7%) had fewer adverse effects and no significant side effects. For total sleep duration, total sleep duration in APP/PS1 (AD) mice (MD=36717,95%CI: 35807 to 37626) seconds, APP/PS1 (AD) mice (MD=2762,95%CI: 1506 to 4018) seconds, and TGN + APP/PS1 (AD) 30 mice (MD = 16,903,95%CI: 15647 to 18160) seconds. In the Y maze and the Morris water maze, TGN and TGN + 30mg mice had higher escape latency than controls, and A β deposition was increased on immunohistochemistry.
Conclusion: Compared with the control group, the TGN and TGN + 30 groups have impaired learning and memory, and strengthening the Aqp 4-led lymphoid drainage function in the brain has important significance for the generation, development and outcome of Alzheimer's disease. Aqp 4 agonists or AQP 4 open agents are expected to be novel targets for the future treatment of Alzheimer's disease.
AQP4 • Alzheimer's disease • Cognition • Learning • Memory
The researchers found that AD patients were accompanied by AQP4 deletion [1]. Aqp4 is the most expressed aquaporin in the brain, which is highly expressed in astrocyte foot processes and around blood vessels, and plays an important role in the circulation of lymphoid pathway [2, 3]. Loss of AQP4 results in a β. The clearance rate of tau and tau protein decreased [4, 5], nerve fiber tangled, and accelerated the formation and progress of AD [6]. This indicates that regulating AQP4 will become a new target to delay the pathological development of AD and reduce cognitive damage [7, 8]. In this experiment, we injected TGN and TGN+DORA into APP/PS1 (AD) mice to explore the effect of AQP4 on A β The intervention effect of protein expression and the possible mechanism of its influence on APP/PS1 (AD) mice's cognitive impairment from the aspects of behavior, histology and molecular biology [9]. Sleep monitoring found that compared with TGN group, TGN+DORA group had a longer sleep time, which was enough to induce sleep and prolong sleep time, further confirming the effectiveness and safety of Dora as a new hypnotic drug in treating sleep disorders [10, 11]. The application of TGN intensifies a β It can be assumed that AQP4 is used to regulate the glial lymphatic system, which can not only serve as a channel for small molecule drug transport, but also increase the proportion of drugs entering the central nervous system [12, 13]. AQP4 agonists can also be used to regulate a β The elimination of other metabolites can delay the progression of neurodegenerative diseases such as cognitive impairment and AD.
Laboratory animals, reagents and instruments
39-month-old healthy male Amyloid Precursor Protein/Presenilin 1 (APPswe/ps1de9, APP/PS1) double transgenic AD mice weighing 28-35 g (animal license No.: sckk (Beijing) 2016-0009) were provided by Beijing Weishang Lide Animal Breeding Co., Ltd. This experiment was approved by the Animal Ethics Committee of Shandong Qianfo Mountain Hospital. An AQP4 blocker (cas-51987-99-6) was purchased from Selleck Company, and a rabbit two-step immunohistochemistry kit was purchased from Beijing Zhongshan Jinqiao Biotechnology Co., Ltd. TGN was dissolved and stored in Dimethyl Sulfoxide (DMSO) solution and diluted with 0.9% double distilled water. A mouse sleep monitor (model: xr-xs107) and Morris water maze (version: Super maze 2.0) were purchased from Shanghai Xinruan Information Technology Co., Ltd.
Grouping and treatment of mice
Forty-one mice were randomly divided into four groups with a computer: the control group; the 30 group, which included mice injected with 30 mg/kg Almorexant (a DORA); the TGN group, which included mice injected with 10 mg/kg TGN (an AQP4 receptor blocker); and the TGN+30 group, which included mice injected with 10 mg/kg TGN and 30 mg/kg Almorexant. There were 11 mice in each group except the control mice, which contained eight mice. The solvent was composed of DMSO, PEG300, TWEEN-80 and normal saline, and the drugs were administered according to mouse body weight and a previously reported experimental design. The control group was injected with solvent only. Drugs were administered by intraperitoneal injection daily during the light phase (i.e., at 6:00 a.m.) for 28 days. A sleep monitor was used to monitor the sleep of mice in the control group, TGN group and TGN+30 group over 24 hours (from 06:00 a.m. on the first day to 06:00 a.m. on the second day), as shown in Figure 1. All mice were kept in the SPF animal laboratory of the First Affiliated Hospital of Shandong First Medical University at a constant temperature and humidity and provided sufficient water and food.

Figure 1: Note- At 6:00 in the morning, the mice were intraperitoneally injected. After the injection, the mice were put into a sleep monitoring instrument to monitor the sleep duration for 28 days. After that, the Y maze experiment was conducted for 2 days, and the water maze experiment was conducted for 7 days. After the behavioral experiment, the mice were killed after anesthesia, and the brain was taken with pbs perfusion for immunohistochemical test A β Deposition.
Sleep monitoring and drug intervention model
A sleep monitor was purchased from Shanghai Xinruan Company. A transparent acrylic cylinder with a diameter of 30 cm and a height of 35 cm was used in conjunction with motor and electronic monitoring software, and the shaft sleeve was located at the bottom of the cylinder. Before monitoring, the mice were kept in the sleep monitor for one week for adaptation to avoid the impact of environmental changes on mouse activity. After drug injection, the mice were placed in the sleep monitor. The parameters were as follows: 24 hours/day, time: 06:00 a.m.-06:00 a.m. the next day). Sufficient amounts of water and food for 28 days were placed in the sleep monitoring instrument. The drugs were prepared in a clean environment, and an appropriate amount of drug was injected into each mouse according to body weight. The drugs were dissolved as follows: 300 mg of drug was dissolved in 2 ml of DMSO, shaken and mixed until the liquid was clear to obtain a 150 mg/ml stock solution in DMSO. The solvent was composed of 5% DMSO, 5% Tween-80, 40% PEG-300 and 50% double distilled water. The drugs were freshly prepared on the day of injection. The control group was injected with the same amount of solvent. The drugs were administered by intraperitoneal injection. All syringes were sterile disposable syringes. Before and after injection, the injection site was disinfected with iodophor to reduce the probability of infection. Within 4 hours after drug injection, the experimenter observed the activities of the mice in their cages, recorded adverse reactions; video recorded the mice, and observed whether the mice showed abnormalities, such as sudden falling, limb weakness, infection, etc.
Determination of the physiological indexes of mice
The two experimenters measured one by one according to the mouse ear mark one day before the start of the experiment and 28 days after the end of the experiment. Fix the mouse with a special instrument and place it on the animal electronic scale in the animal laboratory (weight 0 g-100g, accuracy 0.01g). After measurement, input it into the computer for recording and analysis. The experimenter added food and water to the mice at 12 o'clock every day. Before and after each addition, measure and record the weight of each water bottle and each cage of feed in each group. Finally, the average value and standard deviation are calculated by SPSS software and xlsx tool.
Y-maze test
After 28 days of drug injection, the behavior of animals was tested. Y maze was used to evaluate the short-term working memory of mice, and the Y maze device was purchased from Shanghai Xinsoft Company. It is composed of three identical arms at 120° to each other. Each arm is 35cm long, 5 cm wide and 15 cm high. Different shapes are pasted on the inner side of each arm as visual signs of mice. There is a movable partition at the joint of the three arms. The infrared camera was placed 1.5 meters above the maze to capture the mouse's activities, and the software parameters were adjusted to better track the mouse's trajectory. T maze and water maze experiments were conducted in a quiet and dark environment during the active period of mice. The three arms are set as a (starting arm), B (other arm) and n (new arm) respectively. First, the partition board is installed at the entrance of the N arm, so that the mouse can move freely in the A arm and B arm, and calculate the times and time ratio of mice entering the new arm. The partition was then removed so that the mice could move freely in the three arms. Calculate the percentage of spontaneous alternation of each mouse=total alternation times/maximum alternation times × 100%ã??
Water maze test
After the Y maze experiment, the mice were tested with Morris Water Maze (MWM) to evaluate the long-term spatial learning and memory of the animals. The water maze device was purchased from Shanghai Xinruan Company. The diameter of the pool is 1.2 m, the height is 50 cm, the water surface is 35 cm high, the water temperature is set between 22ºC-24ºC and the diameter of the platform is 12 cm, 1 cm higher than the water surface. Edible titanium dioxide is put into the water to dissolve, so that the underwater platform is hidden. Four black identification marks with different shapes shall be pasted on the four quadrants of the pool wall. The platform was placed in the middle of the second quadrant, and the platform position remained unchanged during the experiment [14]. The swimming pool is covered with opaque curtains, and an infrared camera is placed 1.5 m above the center of the swimming pool. The super maze software was used to record the swimming track of the mice. Morris water maze test included: the positioning navigation test was conducted four times a day to record the time required for the mice to find the platform within 1 min after entering the water at different points (escape latency). If the mice did not find the platform within 1 min after entering the water, they were guided to stay on the platform for 20 seconds, the space exploration test of learning and memory platform of mice was to record the times of mice crossing the platform position within 1 minute after removing the platform.
Immunohistochemistry and fluorescence staining
After the water maze experiment, the mice were anesthetized and perfused, and the brains were collected. Isolated brain tissue was fixed with 4% paraformaldehyde for 24 hours-48 hours. The fixed brain tissue was dehydrated, embedded, and prepared. The tissue was sectioned coronally, dewaxed, subjected to antigen repair, blocked, incubated with primary antibody overnight, incubated with secondary antibody and DAB, stained with hematoxylin, dehydrated, and mounted. After staining, the parameters of the optical microscope were kept constant for observation of all sections. After completion of fluorescence staining, primary antibody diluted with PBS (AQP4, rabbit origin, 1:500, D1F8E, CST; CD31, mouse origin, 1:100, NB10064796SS, Novus Biologicals) was added to evenly cover the brain tissues. The tissues were placed in a cassette at 4°C overnight. The next day, the cassette was removed and rewarmed at room temperature for 30 minutes. The tissues were incubated at 37°C for 30 minutes after the addition of secondary antibody (Cy3-labeled goat anti-rabbit, 1:200, Xavier; FITClabeled goat anti-mouse, 1:100, Xavier). Anti-fluorescence quenching agent (including DAPI) was used to mount the tissues, and the fluorescently stained tissue sections were scanned with an Olympus digital slide scanner (VS2000). Finally, the images were analyzed with Image-Pro Plus software.
Statistical analysis
SPSS 22.0 statistical analysis software was used for statistical analysis of the above experimental data, and GraphPad Prism 8 and Photoshop CC were used for data visualization and image layout. The data in this experiment conformed to a normal distribution and showed uniform variance, and the data are expressed as the mean ± standard deviation. The escape latency data from the positioning navigation phase of the Morris water maze experiment were analyzed by repeated-measured Analysis of Variance (ANOVA) followed by multiple comparisons post hoc tests. Other data were analyzed by independent samples one-way ANOVA followed by the LSD-t multiple comparisons post hoc test. P<0.05 was considered statistically significant.
Body weight change (D) of mice on the 28th day, and food intake change (E) of mice on the 24th hour. The mice on the 24th day recorded drinking water (F)in the experiment on the 28th day, weighed the body weight every 7 days, and recorded food and drinking water every 24 hours. The mice in 60 mg group and TGN+DORA group had the most significant changes. During the intervention period, the weight of mice in each group decreased, and the mortality rate during the administration and modeling period was 8% (5/63), * P<0.05, * * P<0.01.
Total sleep duration
Total sleep was significantly longer in the control group (MD=36717,95%CI: 35807 to 37626) seconds, in APP/ PS1 (AD) mice in the TGN group (MD=2762,95%CI: 1506 to 4018) seconds, and in APP / PS1 (AD) mice in the TGN + 30 group (MD=16903,95%CI: 15647 to 18160) seconds.
Sleep long during the day
Control APP / PS1 (AD) mice had significantly longer daytime sleep duration (MD=28068, 95%CI: 26,797 to 29339) sec, Compared with the control group, The APP/PS1 (AD) mice in the TGN group had a significantly longer daytime sleep duration (MD=1763, 95%CI: 626 to 3519) in seconds, Compared with the control group, The APP / PS1 (AD) mice in the TGN + 30 group had a significantly longer daytime sleep duration (MD=12479, 95%CI: 10,723 to 14236) in sec.
Sleep long in black days
APP / PS1 (AD) mice (MD=8649,95%CI: 7113 to 10184) seconds, APP / PS1 (AD) mice in TGN (MD=12479,95%CI: 1123 to 3121) seconds, and APP / PS1 (AD) mice in TGN + 30 compared with control group (MD=4424, 95%CI: 2,302 to 6546) in seconds. Almorexant Prolong the length of sleep and shorten the time of sleep. During the next 12 hours after injection (from 6 am to 6 pm), the group showed a significant dose-response pattern. In the following 12h (6 p. m. to 6 a. m.), the mice except 60mg / kg group slept 269 ± 133, while the other groups slept less than 4 hours and the difference between the groups was not obvious. At 3 a.m.-6 a. m., the next day, when the mice were active, and the camera observed no significant difference in the activity, sensitivity and alertness of the mice compared with the control group. Suggesting that Almorexant effectively promoted sleep and does not damage daily activities the next day, as shown in Figure 2.
Figure 2: The sleep monitoring instrument runs well, and the sleep monitoring data is reliable and accurate. Compared with TGN group, the activity of mice in TGN+DORA group decreased and their sleep increased after intervention. After injection, the mice in TGN+DORA group fell asleep faster and their total sleep time increased.
Tolerability and safety
Except that the incidence of reaction retardation was 6%, the incidence of other convulsions, hematochezia and paralysis was 1.5%. On day 7, about 5 seconds after the injection of 60mg / kg mice, the mice suddenly lost their muscle tone, and the head dropped and the body tilted to the right side, indicating limb curvature and eyelid paralysis. Related to Almorexant injection, the brain orexin was suddenly blocked in large quantities and unable to maintain normal arousal and basic activity. On day 22, the control group had a mouse bleeding in the stool, and it was speculated that the blood stool was caused by intestinal foreign body scratches. In this experiment, the mortality rate of the mice was 5.1%. After dissection, the dead mice adhered the small intestine, and the intestinal contents passed through obstacles, causing intestinal obstruction and died.
Y maze
New arm exploration time ratio: APP / PS mice 1 (AD) (MD=0.255, 95%CI: 0.189 to 0.320) seconds, Compared with the control group, The APP / PS1 (AD) mice had no significant decrease in the Y-maze time ratio in the TGN group (MD=0.085, 95%CI: 0.175 to 0.006) seconds, Compared with the control group, No significant reduction in the Y-maze time ratio in APP / PS1 (AD) mice in the TGN+30 group (MD= 0.078, 95%CI: 0.169 to 0.012) seconds.
The number of new arm explorations vs Control: APP / PS1 (AD) mice were significantly longer than the Y maze number (MD=0.343, 95%CI: 0.284 to 0.401) seconds, Compared with the control group, APP / PS1 (AD) mice in the TGN group (MD= 0.109, 95%CI: 0.190 to 0.028) seconds, Compared with the control group, APP / PS1 (AD) mice in the TGN + 30 group (MD= 0.076, 95%CI: 0.157 to 0.006) seconds as shown in Figures 3 and 4.
Figure 3: Compared with the control group, the number and percentage of new arm recognition experiments in TGN group and TGN+DORA group mice decreased. Compared with TGN group, TGN+DORA group mice were more inclined to explore new arms. TGN damaged the short-term learning and memory of mice, while the combined application of 30 mg/kg DORA seemed to slightly reduce the cognitive impairment of mice.
Figure 4: Typical mouse movement track (A), mouse swimming speed (B), times of crossing platform (C), target quadrant percentage (D), escape latency (E). The mice in TGN group tended to rotate irregularly along the edge of the pool and appeared to swim along the edge. The mice in con group tended to find the platform position more quickly and accurately. The mice in TGN and TGN+DORA groups did not perform as well as those in con group, showing a certain degree of learning and memory impairment.
Spontaneous alternation ratio: The Y maze of the control APP / PS1 (AD) mice (MD=0.407, 95%CI: 0.337 to 0.477) seconds, Compared with the control group, APP / PS1 (AD) mice in the TGN group (MD= 0.143, 95%CI: 0.240 to 0.046) seconds, Compared with the control group, APP / PS1 (AD) mice with TGN +30 significantly decreased the percentage of spontaneous Y maze alternation (MD= 0.098, 95%CI: 0.195 to 0.001) seconds.
Morris water maze
Water maze time ratio: Control APP / PS1 (AD) mice had a significantly longer water maze time ratio (MD=3.1, 95%CI: 2.356 to 3.844) seconds, Compared with the control group, APP / PS1 (AD) mice in the TGN group (MD= 1.191, 95%CI: 2.219 to 0.163) seconds, Compared with the control group, APP / PS1 (AD) mice in TGN + 30 (MD= 0.736, 95%CI: 1.764 to 0.291) sec.
The number of water maze ratio: The APP / PS1 (AD) mice in the control group had a significantly longer water maze times ratio (MD=0.295, 95%CI: 0.252 to 0.338) seconds, Compared with the control group, APP / PS1 (AD) mice in the TGN group (MD= 0.321, 95%CI: 0.191 to 0.073) seconds, Compared with the control group, APP / PS1 (AD) mice in the TGN + 30 group (MD=0.126, 95%CI: 0.185 to 0.067) seconds.
Immuno-histochemical
Cortical area: % A β plaque positive area change in APP / PS1 (AD) mice (MD=0.185, 95%CI:0.174 to 0.195), Compared with the control group, A β plaque positive area area in the TGN group (MD=0.016, 95%CI:0.003 to 0.004), Compared with the control group, No significant reduction in A β plaque positive area in the TGN + 30 group (MD= 0.001, 95%CI:0.014- 0.014).
Hippocampal area: % Significant change of A β plaque positive area in control APP / PS1 (AD) mice (MD=0.181, 95%CI:0.173 to 0.188), Compared with the control group, In APP/PS1 (AD) mice in the TGN group (MD=0.014, 95%CI:0.003 to 0.024), Compared with the control group, A β% of plaque positive area area increase in TGN + 30 (MD= 0.031, 95%CI:﹣0.041 to 0.198) as shown in Figure 5 and 6.
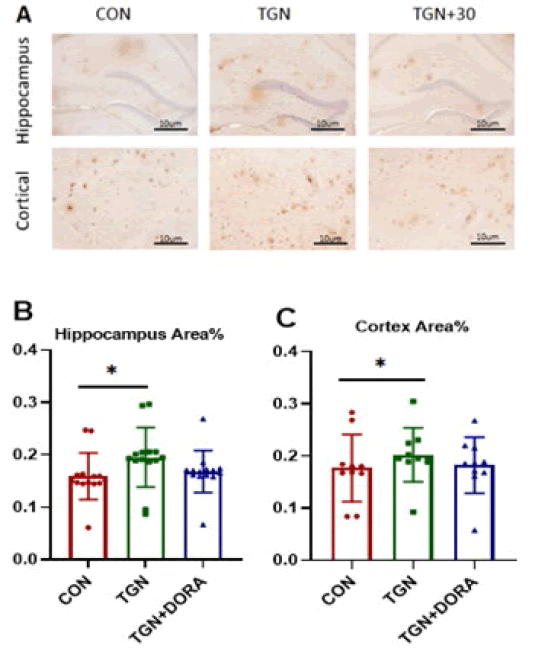
Figure 5: Aβ typical plaque immunohistochemical staining (A) cortical area A β Semi quantitative analysis (B), hippocampus A β Semi quantitative analysis (C): TGN can increase A in app/ps1 mice β Deposition, *P<0.05, * *P<0.01、
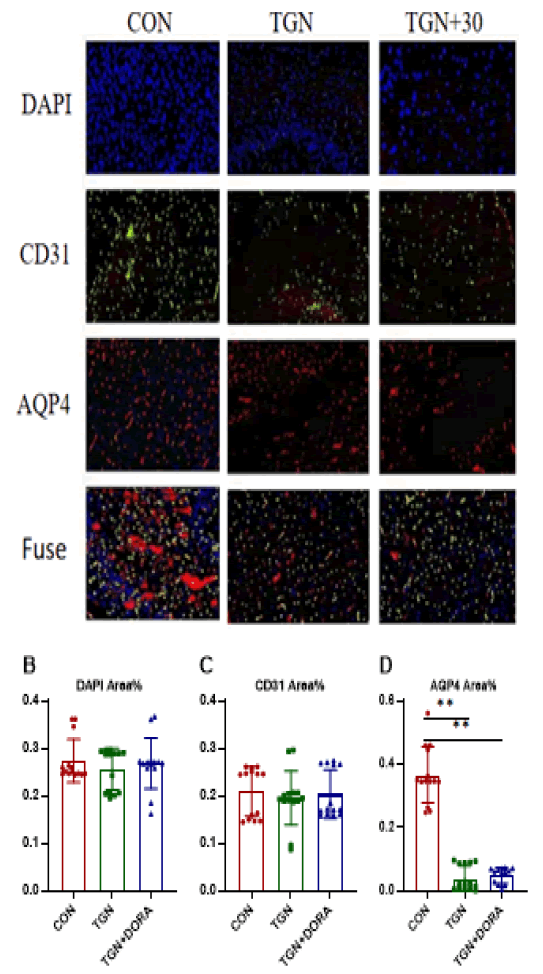
Figure 6: In typical immunofluorescence staining pictures, blue represents DAPI, green represents CD31, and red represents AQP4. Compared with con group, TGN and TGN+DORA groups are effective in intervention and significantly block the deposition of AQP4.
AQP4 is mainly expressed on astrocyte foot processes, which constitute the blood brain barrier, and regulates the water balance in the brain. A small portion of AQP4 is distributed on ependymal cells [15, 16]. AQP4-mediated transport of water molecules is independent of the membrane potential and occurs in an unsaturated manner; thus, it can increase the permeability of the cell membrane to water by several times to tens of times [17, 18]. Soluble Aβ exists in the brain parenchyma. There are two main mechanisms of the clearance of β-lactamase: the paravascular pathway and glial activation. Aβ was found in the brain parenchyma of mice injected with an AQP4 blocker. The clearance rate was reduced by approximately 70% [19, 20]. AQP4 knockout mice exhibit increased Aβ deposition, which is caused by dysfunction of glymphatic system [21, 22]. In this experiment, mice in the TGN-treated groups showed few adverse reactions, the most common of which was reduced activity, which was observed in the TGN and TGN+30 groups. In the study, Aβ deposition in the hippocampus was increased in the TGN and TGN+30 group mice. The percentage of spontaneous alternations in the Y maze and the ratio of time spent in the target quadrant in the Morris water maze were increased, and the escape latency in the hidden platform test was decreased. A decrease in AQP4 expression causes a decrease in glymphatic circulation and the expression of proteins such as Aβ42 in the brain. Abnormal deposition of Tau can aggravate neurocognitive impairment and persistent neuroinflammation in AD. In addition, during sleep, the cerebral cortex integrates and processes short-term and long-term memories, forming new synaptic connections, which is conducive to the effective recovery of energy [23, 24]. Loss of AQP4 reduces the integration of newborn neurons into learning- and memory-related neural circuits in adults, impairs memory for objects in specific locations, and affects the acquisition and storage of memory [25-27]. Similarly, over activation of orexin may also allow damage to the lymphatic system by Aβ and other types of metabolic waste, leading to extracellular Aβ and increased tau deposition and NFT formation, which results in neuronal dysfunction and deterioration of cognitive function [28, 29]. In conclusion, in this study, TGN aggravated learning and memory impairment in APP/PS1 mice. However, in the TGN+30 group, 30 mg/kg DORA increased the number of platform crossings and the percentage of time spent in the target quadrant in the Morris water maze, potentially alleviating learning and memory impairment in the mice to some extent. TGN reduced Aβ clearance, hindered Cerebrospinal Fluid/Interstitial fluid (CSF/ISF) exchange, and accelerated cognitive impairment and neurodegeneration in AD. The polar distribution of AQP4 and Aβ is altered in the brains of AD patients. The accumulation of harmful substances such as Tau protein and a decrease in the lymphatic clearance rate lead to cognitive dysfunction [30, 31]. An increasing number of researchers have begun to pay attention to the role of AQP4 in the pathological process of AD, and we can assume that using AQP4 to regulate the glymphatic system can not only improve small–molecule drug transport but also increase the proportion of drugs that enter the central nervous system. AQP4 agonists can also be developed to regulate Aβ clearance. AQP4 agonists can delay cognitive impairment and the progression of neurodegenerative diseases such as AD.
Compared with the control group, the TGN and TGN + 30 groups have impaired learning and memory, and strengthening the AQP 4-led lymphoid drainage function in the brain has important significance for the generation, development and outcome of Alzheimer's disease. AQP 4 agonists or AQP 4 open agents are expected to be novel targets for the future treatment of Alzheimer's disease.
We thank all authors who contributed data to our analyses and the foundations and committees that supported this study. Thank you for language assistance during the preparation of this manuscript. Two authors participated independently in the design, implementation, and evaluation of the article and had formal training.
The article was scrutinized with a professional anti-plagiarism literature detection system prior to publication. The statistical methods in the article were approved through a statistical expert review by Shandong First Medical University Graduate School Biology. This is an open access article which, under the terms of the Creative Commons license (4.0) (attribution 1 noncommercial share identical by descent), permits others to edit, adapt, and expand on the original article for noncommercial purposes, provided that the article is read, downloaded, copied, transmitted, printed, retrieved, hyperlinked by any user, and building for indexing, to be used as input data to the software or for any other legitimate use.
Funding was provided by the National Natural Science Foundation of China (based on orexin-A signal transduction characteristics to explore the molecular and cellular mechanisms of sleep deprivation impairing learning and memory, 81471345), the Natural Science Foundation of Shandong Province (Research on the effects of sleep disturbances on AD learning and memory and their molecular and pathological mechanisms, ZR2020MH160), and the Shandong University Horizontal Program (Comparative Study on Early Intervention of Sleep Disorders to Delay Alzheimer's Disease, 089/2019 horizontal).
Citation: Zhou, M., et al. Effect of TGN-020 on Learning and Memory in 8-month-old APP/PS1 (AD) Mice. J Neuro Neurophysiol 2023, 14 (7), 001-006
Received: 28-Mar-2024, Manuscript No. jnn-23-104130; Editor assigned: 29-Mar-2024, Pre QC No. jnn-23-104130(PQ); Reviewed: 07-Apr-2024, QC No. jnn-23-104130(Q); Revised: 09-Apr-2024, Manuscript No. jnn-23-104130(R); Published: 16-Apr-2024, DOI: 10.35248/2332-2594.23.14 (7).341
Copyright: ©2023 Zhou, M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.