Review Article - (2021) Volume 0, Issue 0
Current advents of new technology in prostate cancer treatment still carries many problems regarding oncological outcome: Local control remains elusive, with biochemical failure ultimately occurring in half of the patients diagnosed by PSA screening. Failure of the initial treatment imposes many kinds of physical, psychological, and economical burdens on the patients, such as toxicities caused by salvage hormonal therapy or radiotherapy, risk for developing Castration-Resistant Prostate Cancer (CRPC), and financial insecurity after second or third salvage drug therapy
Considering these potential adverse effects, physicians should conduct prostate cancer treatment with the utmost care and attention to details. In order to minimize local recurrence the author has invented a novel technique, called the “Ten-Step Method,” for seed implantation that can disseminate quality high-dose radiation. In this review, the author summarizes technique, application and oncological outcome of the high-quality high-dose, Low- Dose-Rate (LDR) branchy therapy that can minimize local recurrence of prostate cancer.
Low-Dose-Rate (LDR) • Brachytherapy • Seed implantation
The diagnostic frequency of no metastatic prostate cancer has increased rapidly after the broad adoption of Prostate-Specific Antigen (PSA) screening [1]. Most of these patients select radical treatments, but those with low-risk prostate cancer prefer active surveillance. Nguyen, et al. warned that newer and costlier prostate cancer therapies were rapidly and widely adopted, causing excess national spending without thorough comparative effectiveness research [2]. Ten years later, this trend continues with the advent of robotic surgery and proton beam radiotherapy [3]. Despite the development of new technology, local control remains elusive, with biochemical failure ultimately occurring in 40% to 60% of the patients [4].
In order to disseminate our rationale and the skills necessary to avoid prostate cancer recurrence, the author has summarized the technique and outcome of our high-quality, Low-Dose-Rate (LDR) brachytherapy (seed implantation), which may be the ultimate radiotherapy for patients with intermediate-risk, high-risk, and super-high-risk prostate cancer, including pelvic lymph node metastasis [5-8].
Clinical problems in current prostate cancer treatment
As described in the introduction, many problems exist in the current prostate cancer treatment modalities that can result in treatment failure. The author describes the reasons for treatment failure after radical prostatectomy and external beam radiotherapy, which are the most common treatment modalities at this time. Among the prostate cancer risk categories described by the NCCN (National Comprehensive Cancer Network), this review excludes low-risk prostate cancer, because patients with low-risk prostate cancer can be safely managed by active surveillance [8]. Thus, in this review, the author focuses on intermediate- and high-risk prostate cancer.
Problems in radical prostatectomy
Radical prostatectomy has been one of the standard treatment modalities for non-metastatic prostate cancer. Grimm, et al. wrote a large comprehensive review of the literature comparing risks for patients stratified by treatment options and with long-term follow-up [9]. According to this literature review, the Biochemical Failure-Free Survival (BFFS) rates at 8 years after radical prostatectomy were 70% and 40% in patients with intermediate-risk and highrisk prostate cancer, respectively [9]. The BFFS rate in radical prostatectomy has not been improved by the advent of robotic surgery [10-13].
The reason for the unsatisfactory BFFS rate in radical prostatectomy is obvious: due to the local anatomy, surgeons cannot secure a sufficient surgical margin as compared with surgery for rectal cancer or other types of cancer (Figure 1). As predicted in a nomogram [14-16], preoperative organ-confined disease has often been diagnosed as non-organ-confined disease after prostatectomy. Upstaging is commonly observed after radical prostatectomy [17,18]. The upstaging or surgical margin positivity in prostatectomy specimen may result in insufficient excision of prostate cancer: This situation exposes the patient to the risk of prostate cancer recurrence. After all, a long history of outcomes of radical prostatectomy tells us that prostate cancer should be treated beyond the prostatic capsule: Namely, an optimal treatment for prostate cancer is a modality that can be applied outside the area of the prostatic capsule.
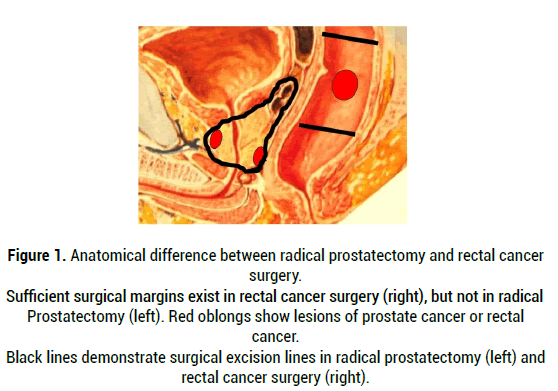
Figure 1: Anatomical difference between radical prostatectomy and rectal cancer surgery. Sufficient surgical margins exist in rectal cancer surgery (right), but not in radical Prostatectomy (left). Red oblongs show lesions of prostate cancer or rectal cancer. Black lines demonstrate surgical excision lines in radical prostatectomy (left) and rectal cancer surgery (right).
Problems of external beam radiotherapy
The same type of problems has been observed in External Beam Radiotherapy (EBRT): So-called high-technology EBRT equipment has been widely adopted, including Intensity-Modulated Radiation Therapy (IMRT), helical tomotherapy, proton beam radiotherapy and carbon ion radiotherapy. Grimm, et al. reported BFFS rates at 8 years after EBRT of 70% and 50% in patients with intermediate-risk and high-risk prostate cancer, respectively [9].
Apart from type of equipment, two key factors are essential to avoid local recurrence in radiotherapy:
1) A sufficiently high radiation dose and
2) Radiation exposure of the entire prostate without cold spots (insufficiently irradiated areas in the prostate).
Concerning factor (1), the author and others have advocated that the requited Biologically Effective Dose (BED) (α/β ratio=2) is 200Gy in intermediaterisk prostate cancer and 220Gy in high-risk prostate cancer [5-8,19,20].
Stone, et al. have shown that patients with Gleason 8-10 disease receiving a Biologically Effective Dose (BED) ≥ 220Gy by combination therapy with Low-Dose-Rate (LDR) brachytherapy and EBRT, obtained improvement in Biochemical Failure-Free Survival (BFFS) [19]. Regarding BED, recent representative BEDs by new EBRT equipment were 157Gy by IMRT [21], 156Gy by helical tomotherapy [22], 148Gy by proton beam radiotherapy [23], 161Gy by carbon ion radiotherapy [24]. All of these modalities failed to achieve the optimal BED proposed by this author and others [5-8,20] . A second problem, which may result in development of cold spots, arises because the prostate is an organ in continuous motion during EBRT: This problem is relevant to the factor (2). Recently, Image-Guided Radiotherapy (IGRT) equipment has been developed: Although clinical implementation of IGRT has improved treatment accuracy and margin reduction in EBRT, it is still uncertain whether the dosimetry and geometric gains can actually improve clinical outcomes [25]. As described later, seed implantation is the ultimate modality in view of motion tracking. Implanted seeds are moving with the prostate: That is obviously far better than motion-tracking EBRT. Thus, there remains a lot of uncertainty regarding radiation dose and cold spots due to prostate motion, although many institutions have adopted newly developed EBRT equipment. A final concern regarding the outcome using the newly developed EBRT equipment is the length of hormonal therapy. NCCN recommends 4-6 months of Androgen Deprivation Therapy (ADT) for patients with intermediate-risk prostate cancer and 2-3 years of ADT for patients with high-risk prostate cancer in the EBRT protocol. Usage of long-term ADT should have a strong impact on data analysis of the BFFS rate. Spiegel, et al. made a nomogram of Testosterone Recovery (TR) after cessation of ADT [26]. They reported that the median time for TR after cessation of ADT is 24 months for patients who received two years of ADT. Tomita, et al. reported the outcome of helical tomotherapy [22]. By applying helical tomotherapy, they have demonstrated BFFS rates at 5 years of 98.2% and 97.7% in patients with intermediate- and high-risk cancer, respectively. However, this report contains an inherent analytical pitfall. In intermediate-risk patients they used 10 months neoadjuvant and 19 months adjuvant ADT: Median durations of ADT were 29 months and 30 months in intermediate- and high-risk patients, respectively. According to Tsumura, et al. [27] more than half of the patients who received long-term ADT did not experience Testosterone Recovery (TR) at 5 years after cessation of ADT. Moreover, one-fifth of the patients who received long-term ADT still had castration levels of testosterone at 5 years after cessation of ADT. The report by Tomita, et al. defined the start date of follow-up as the start of EBRT [27]. This means that after the cessation of ADT at 5 years the period of follow-up would be only 28 months. Similarly, Kawamura et al. reported the outcome of carbon ion radiotherapy [24]: By applying carbon ion radiotherapy, they have demonstrated BFFS rates at 5 years of 92% in high-risk patients. They administered 5-8 months of neoadjuvant ADT and 24 months of adjuvant ADT, respectively, before and after carbon ion radiotherapy. This means that after the cessation of ADT at 5 years the period of follow-up would be only 36 months. Therefore, this study also may only represent the continuing effect of long-term ADT. Without using long-term ADT, Abu-Gheida, et al. reported that the IMRT outcome with regard to the BFFS rate was 63% in patients with high-risk prostate cancer at 5 years [21]. In conclusion of this section, the BEDs of the current new EBRT modalities are not high enough to eradicate intermediate- and high-risk prostate cancer. Use of long-term ADT may produce a good clinical outcome due to the continuing effect of long-term ADT.
What is quality Low-Dose-Rate (LDR) brachytherapy?
Intermediate-risk prostate cancer patients represent the largest of the risk groups and comprise a heterogeneous population of patients with variable prognoses [28].
Based on this heterogeneity, a new classification subdividing patients with intermediate-risk prostate cancer into “favorable” and “unfavorable” subgroups has been proposed:
1) Favorable Intermediate-Risk (FIR): one IR factor with Gleason score 3+4
2) Unfavorable Intermediate-Risk (UIR): Gleason 4+3=7 or >1 intermediaterisk factors (cT2b, cT2c, PSA 10-20, Gleason 3+4=7)
A group at Johns Hopkins University reported on BFFS in a cohort of 4,164 intermediate-risk patients [29]. The results showed that 5-year BFFS differed significantly between FIR patients and UIR patients. For patients with one intermediate-risk factor, the 5-year BFFS was 83.0%, compared with 64.3% for men with two risk factors and 45.9% for those with three risk factors [29] . Similar differences in BFFS between FIR patients and UIR patients have been reported for EBRT of 81Gy [30]. In this report, the estimated 8-year BFFS rates were 86.1% and 71.1% in FIR patients and UIR patients, respectively [30]. The author of this review has shown excellent clinical outcomes with Low-Dose-Rate (LDR) 125 I seed implantation-based radiotherapy for intermediate-risk patients, including a substantial number of UIR cases. BFFS rate of 99.1% at 7 years [6]. In that study, the author concluded that LDR "125I brachytherapy" (seed implantation) alone with a BED of 200Gy is an effective treatment in intermediate-risk prostate cancer, including UIR cases, thus supporting the above-mentioned data from the Mount Sinai group [6]. This unparalleled BFFS rate achieved by LDR monotherapy is based on the “Development of quality high-dose (BED of 200Gy) LDR method”: The details of the technique have been published as the “Ten-Step Method,” which provides implementation specifics for disseminating safe and reproducible high-dose brachytherapy. As described in the section on problems in radical prostatectomy, treatment of prostate cancer should include the space outside of the prostatic capsule. Figure 2 shows a representative radiation dose distribution in LDR monotherapy "125I brachytherapy" in intermediate risk prostate cancer without using ADT by the Ten-Step method (Figure 2).
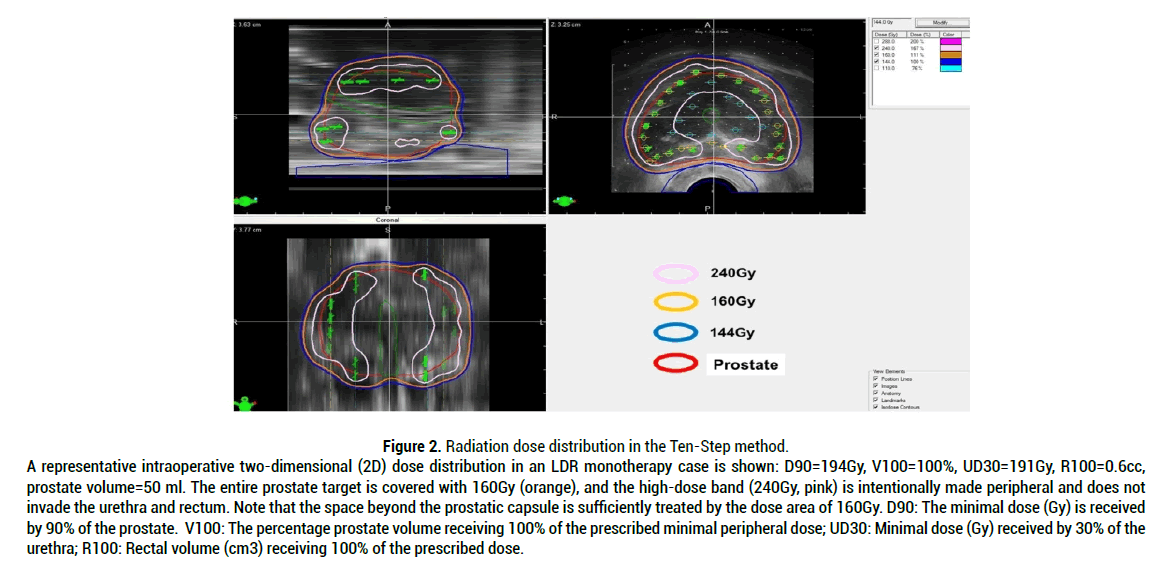
Figure 2: Radiation dose distribution in the Ten-Step method. A representative intraoperative two-dimensional (2D) dose distribution in an LDR monotherapy case is shown: D90=194Gy, V100=100%, UD30=191Gy, R100=0.6cc, prostate volume=50 ml. The entire prostate target is covered with 160Gy (orange), and the high-dose band (240Gy, pink) is intentionally made peripheral and does not invade the urethra and rectum. Note that the space beyond the prostatic capsule is sufficiently treated by the dose area of 160Gy. D90: The minimal dose (Gy) is received by 90% of the prostate. V100: The percentage prostate volume receiving 100% of the prescribed minimal peripheral dose; UD30: Minimal dose (Gy) received by 30% of the urethra; R100: Rectal volume (cm3) receiving 100% of the prescribed dose.
The two-dimensional dose distribution demonstrates that a dose cloud of 160Gy covers the entire prostate and a significant margin beyond the prostatic capsule [8]. This exactly fulfils the author’s proposal that prostate cancer should be treated beyond the prostatic capsule. The method is composed of ten steps, beginning with the positioning of the ultrasound probe and ending with the last seed placement at the midline of the apex between the urethra and rectum. The final step is a confirmation of the dose cloud and dosimetry.
The Ten-Step method is based on the following rationale. First of all, placing a sufficient number of seeds in the peripheral region away from the urethra and rectum insures a good treatment margin beyond the prostate capsule and achieves the following four goals.
1. The entire prostate should be covered by the prescription dose cloud with a sufficient margin (5-7 mm) from the capsule in all directions except for those anterior to the rectal wall, thus achieving high D90 (D90>190Gy) and V100 (V100>99%) values by the "125I seed implantation".
2. A high-dose cloud (240Gy) should not approach the urethra or rectum.
3. In order to achieve goals (1) and (2), it is necessary to make the high-dose cloud intentionally along the periphery (bilateral wall to anterior wall) away from the urethra and rectum.
4. In order to achieve goal (3), seeds at the periphery, except those anterior to the rectal wall, should be placed just 1 mm inside the capsule. The above-mentioned rationale is shown in Figure 3 [8]. By using high dose LDR monotherapy with a BED of 200Gy, patients with intermediate-risk cancer, including UIR, have demonstrated a biochemical failure rate of only 1%. The advantage of this method is the cost-effectiveness for the patients. The patients can omit supplemental EBRT. This can reduce the length of treatment and eliminate the cost of supplemental EBRT. Furthermore, the method does not require hormonal therapy in most cases because the implant quality and dose are consistently high even in cases with large prostates [8].
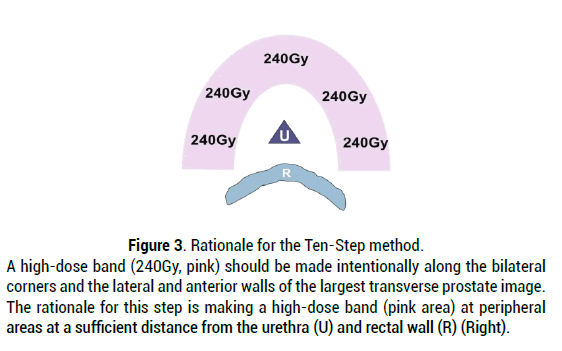
Figure 3: Rationale for the Ten-Step method. A high-dose band (240Gy, pink) should be made intentionally along the bilateral corners and the lateral and anterior walls of the largest transverse prostate image. The rationale for this step is making a high-dose band (pink area) at peripheral areas at a sufficient distance from the urethra (U) and rectal wall (R) (Right).
Quality LDR brachytherapy for high-risk and super-high-risk prostate cancer including pelvic lymph node metastasis
The author has shown good clinical outcomes by escalating the radiation dose for high-risk and very-high-risk prostate cancer patients including five pelvic lymph node metastasis cases with a BFFS rate of 95.2% at five years by tri-modality therapy of BED>220Gy (a combination of LDR brachytherapy, EBRT, and Androgen Deprivation Therapy (ADT) [5]. In this report, 63% of the cases analyzed were T3a, T3b or T4. In this study, we used short-term ADT (six months ADT before and after LDR brachytherapy). The author has also shown that T4 prostate cancer with pelvic lymph node metastasis can be cured in the same way [7]. In the above-mentioned reports, duration of followup started from the end of EBRT. This means that during the 5 years of followup, the period of ADT was only three months: In the studies, representative patients demonstrated that TR decreased to normal range at 9-12 months after cessation of ADT [5,7]. Therefore, the effect of ADT in the reports is negligible. In concluding this section, the studies by the author have shown that patients having locally advanced prostate cancer with pelvic lymph node metastasis can be cured by the author’s strategy using tri-modality with BED of 220Gy.
Strategy for high-risk and very-high-risk prostate cancer patients including pelvic lymph node metastasis cases
In order to achieve a BED of 220Gy in high-risk and very-high-risk prostate cancer patients, including pelvic lymph node metastasis cases, the author also applies the Ten-Step method in seed implantation. Dose distributions of 240Gy, 160Gy, and 144Gy by LDR monotherapy correspond to 160Gy, 130Gy and 110Gy by LDR in combination with EBRT. Seeds should be implanted in the same way by the Ten-Step method. When the author treats cases with super high risk, including pelvic lymph node metastasis or PSA 40 ng/ml, the target area for EBRT is expanded to include the Whole Pelvis (WP) [5,7]. The radiation dose to the WP should be set at 45Gy in order to minimize GI toxicity (Usually 1.8Gy/fraction × 25 times). In order to secure a radiation dose of 45Gy to WP, D90 of LDR should be ranged from 130Gy to 138Gy on the dosimetry schedule at one month after seed implantation. In order to achieve a BED of 220Gy on the prostate, one or two fractions of EBRT to prostate and seminal vesicles only may be added after calculation of BED by LDR. In the other high-risk cases, the target of EBRT is designed to include prostate and seminal vesicles only. In those cases, D90 of LDR should be ranged from 135Gy to 145Gy at one month after seed implantation. Fractions of EBRT can be reduced according to the D90 of LDR at one month after seed implantation and calculation of BED by LDR.
Importance of seminal vesicle seed implantation
In order to conduct quality seed implantation in combination with EBRT in high-risk and very-high-risk prostate cancer patients, the author commonly applies seminal vesicle implantation. Stock, et al. published clear evidence that dose from implanted seeds from the prostate contributes little to the seminal vesicle. Therefore, they proclaimed necessity of seminal vesicle seed implantation for some of the high-risk prostate cancer patients [31]. The author and others have already described the method for seminal vesicle seed implantation [7,32]. A representative dose distribution with seminal vesicle implantation in high-risk or super-high-risk prostate cancer is shown in Figure 4.
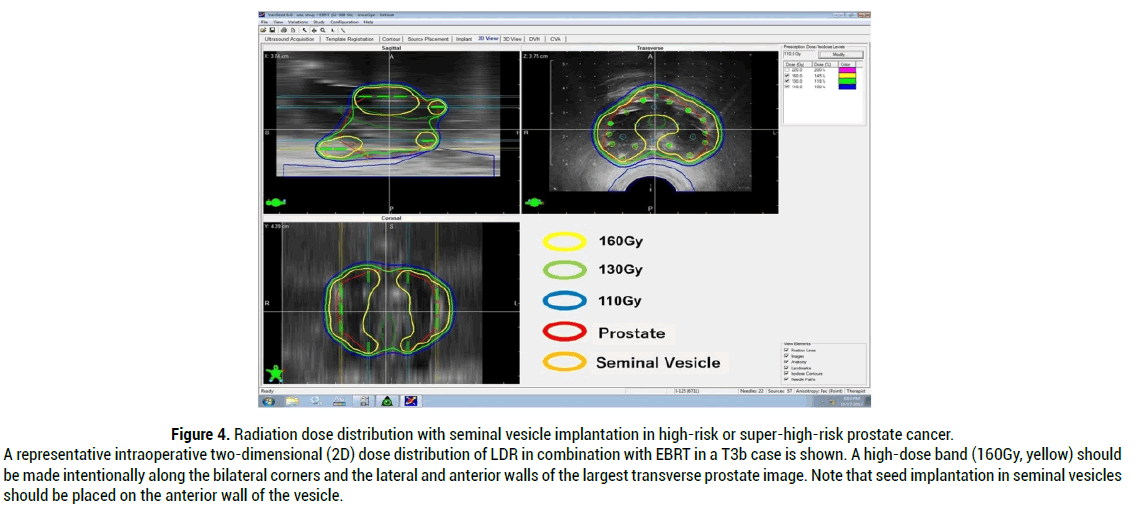
Figure 4: Radiation dose distribution with seminal vesicle implantation in high-risk or super-high-risk prostate cancer. A representative intraoperative two-dimensional (2D) dose distribution of LDR in combination with EBRT in a T3b case is shown. A high-dose band (160Gy, yellow) should be made intentionally along the bilateral corners and the lateral and anterior walls of the largest transverse prostate image. Note that seed implantation in seminal vesicles should be placed on the anterior wall of the vesicle.
Seminal vesicle implantation has two benefits:
1) Treating seminal vesicle invasion.
2) In cases of small-volume prostate, by using neoadjuvant ADT a high D90 is achievable by seminal vesicle seed implantation, which can help in obtaining a high D90 of 130-140Gy without increasing urethral and rectal doses.
In concluding this section, the technique of seminal vesicle implantation is essential for conducting quality seed implantation in combination with EBRT in high-risk and very-high-risk prostate cancer patients.
The author raised the problems associated with current treatments of prostate cancer using newer and costlier modalities. Quality seed implantation by the Ten-Step method can be the ultimate radiosurgery for non-metastatic prostate cancer including very-high-risk prostate cancer with pelvic lymph node metastasis.
Keisei Okamoto was associated with the Department of Brachytherapy for Prostate Cancer endowed by Nihon Medi-Physics Co.,Ltd. The present affiliation of Keisei Okamoto is Uji Hospital, Department of Urology, Kyoto, Japan.
Citation: Okamoto K. Emergence of Quality Low-Dose-Rate (LDR) Brachytherapy: Ultimate Radiosurgery for Non-Metastatic Prostate Cancer. Med Rep Case Stud, 2021, 06(S4), 225
Received: 06-Sep-2021 Published: 27-Sep-2021, DOI: 10.35248/2572-5130.21.s4.001-004
Copyright: © 2021 Okamoto K. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : NO