Mini Review - (2021) Volume 6, Issue 6
Low-grade triple-negative breast carcinomas is a descriptive term that could be used as a diagnostic term in a core needle biopsy if a specific subtype may not be established, referring the definitive diagnosis to the study of the surgical specimen. A correct diagnosis of a specific histopathological subtype is possible in certain cases and it allows adequate treatment within a multidisciplinary breast-unit in an increasingly frequent, neoadjuvant regimens context. It is essential to distinguish clearly between low-grade triple-negative breast cancer and others, as they have a different prognosis and require different therapeutic management during its evolution as a disease.
Low-grade triple-negative breast carcinomas are negative for estrogen receptors, progesterone receptors and HER2 (Human Epidermal Growth Factor Receptor 2), they have low proliferative indexes and a characteristic morphological and immunohistochemically profile.
They show a great molecular heterogeneity and stablishing the transcriptional or intrinsic subtypes is not yet included in daily clinical practice. As a group, they have a relatively indolent course; despite the fact they usually show poor responses to conventional chemotherapy. For this reason, the main problem with this group of tumors consists of not recognizing it as such and applying an inadequate treatment, especially an ineffective chemotherapy without an adequate cost/benefit ratio.
Although there is not enough evidence in some subtypes, it seems reasonable to consider these tumors as a group with characteristic genetic alterations and low response rates to conventional chemotherapy, partly due to a low proliferative index. Surgical treatment could be curative in certain cases, especially those cases diagnosed in early stages. Conventional chemotherapy would not be used in those cases that will not going to obtain enough benefit, doing less harm to the patient. Furthermore, the detection of the expression of some biomarkers (PDL1, NTRK) could be used to carry out a targeted treatment. Finally, the metaplastic subtype is radiosensitive.
Breast cáncer • Tumors • Prognosis • Chemotherapy
Low-grade Triple-Negative Breast Carcinomas (low-grade TNBCs) are characterized by negativity for estrogen receptors, progesterone receptors and HER2 and, although they usually show poor responses to conventional chemotherapy, have a relatively indolent course [1,2].
Low-grade TNBC and diagnosis in core needle biopsy
Low-grade TNBC, as a group, it is constituted of different tumor subtypes: (1) Low-grade carcinomas, which include the Acinic cell carcinoma and several subtypes of the metaplastic carcinoma. Which, in spite of showing the usual TNBC complex genomic landscape, have a low-grade morphology and a good prognosis and (2) carcinomas with similar morphology to those of the salivary glands which are salivary-like carcinomas (i.e., Adenoid cystic carcinoma, Secretory carcinoma, Mucoepidermoid carcinoma, Polymorphic adenocarcinoma, Tall cell carcinoma with reversed polarity, Adenomyoepithelioma) showing characteristic genetic changes, lack of repeated TP53 mutations and low levels of genetic instability [3-12]. These neoplasms, as a group, are difficult to diagnose in Core Needle Biopsy (CNB). This may be due to their rareness and that its morphological characteristics of a low grade-TNBC may not be reflected in the CNB. In order to identify all cases of low-grade TNBC in the context of a triple negative tumor with low or intermediate Ki67 proliferative index (<30%), an extensive panel of antibodies may enhance the accuracy of the histopathological diagnosis: AR, S100, SOX10, GATA3, GCDFP15, mammaglobin, CD117, p63, p53, SMA, chromogranin, synaptophysin, EMA, lysozyme, A1AT and NTRK, as previously have shown [13].
Low-grade TNBC, prognosis and therapeutics implications
TNBCs show a great heterogeneity (Figure 1). At a genetic level may belong to different intrinsic subtypes: basal-like, claudin-low; even enriched HER2 (Human Epidermal Growth Factor Receptor 2) or luminal B. At a transcriptomic level, they may belong to any of the six Lehmann subtypes: Basal-like 1, Basal-like 2, Immunomodulatory, Mesenchymal, Mesenchymal stem-like and Luminal androgen, each one of them with different characteristics and degrees of response to standard neoadjuvant chemotherapy [14-16]. Basallike, some of them are low-grade TNBC, sensitive to platin-based regimens, with 50% of them evolving into pathological complete response after standard neoadjuvant chemotherapy. Most of the Basal-like 2 tumors are low-grade TNBC and, although they are sensitive to platin-based regimens, they showed 0% in pathological complete response after standard neoadjuvancy. Mesenchymal most belong to the metaplastic subtype, and some of them are low-grade TNBC. Luminal androgen receptors, some of them are low-grade tumors, with a 10% of pathological complete response after conventional neoadjuvant therapy, but sensitive to antiandrogenic therapy [17].
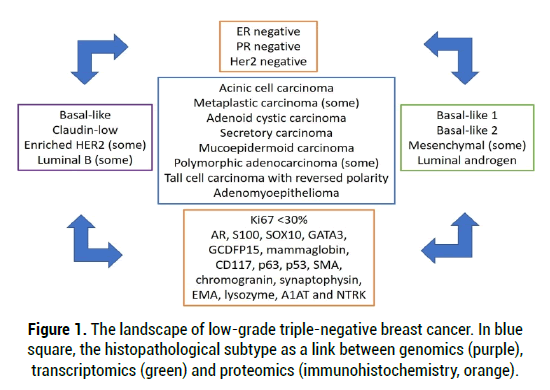
Figure 1: The landscape of low-grade triple-negative breast cancer. In blue square, the histopathological subtype as a link between genomics (purple), transcriptomics (green) and proteomics (immunohistochemistry, orange).
Other taxonomies are also available. However, not all the TNBC transcriptional subtypes (nor all the intrinsic genetic subtypes) are stable or can be identified in a repetitive way [18-20]. Thus, there is a blurring of boundaries between the groups, depending on the selected taxonomy. In addition, some histopathologic subtypes, such as the cystic adenoid carcinoma, or the tall cell carcinoma with reversed polarity, are not represented either in the intrinsic or the Lehmann classification [16]. Moreover, none of the different multigenetic platforms, (Mamaprint, Oncotype, PAM50, Endopredict), developed for predicting the outcomes of chemotherapy, have included any TNBC in their design [21]. Establishing the transcriptional or intrinsic subtypes is not yet included in daily clinical practice and, even if it were, it could prove challenging due to the degree of overlapping of categories and the lack of a widely accepted model for the transcriptional classes.
However, the international histopathological classification groups together neoplasms with common genetically and phenotypical characteristics within the same histopathological subgroup; the resulting morphological patterns are indicators of both treatment and prognosis and should be carefully identified [3].
Due to the characteristic phenotype-genotype association, subtype is an independent prognostic factor, which distinguishes low-grade TNBC from other types, based on the morphological and immunophenotypical characteristics and may even predict the therapeutic response, as happens with secretory carcinoma [22]. The majority of secretory carcinomas present as T1-T2, 20%-35% of them with axillary involvement. Usually, it follows an indolent course, with a postoperative survival rate of 5 and 10 years of 94% and 91% respectively, with prolonged survival even in the presence of lymph node and distant metastases [23,24]. NTRK inhibitors offer a therapeutic alternative in the rare cases of metastases and chemotherapy resistant tumors such as mammary secretor carcinomas [25]. Although the overall survival rate of metaplastic carcinoma, after 3 and 10 years is of 77% and 53% respectively, some patterns are related to a good prognosis: low-grade adenosquamous, fibromatosis-like and with heterologous mesenchymal differentiation. There is lower axillary involvement and a higher tendency for hematogenous dissemination compared with other IBC and radiotherapy provides clinical improvement [3]. The therapeutic approach for acinic cell carcinoma tends to be similar to that for IBC, although the long term prognosis is not clear [4]. Low-grade cystic adenoid carcinomas usually have a good prognosis, especially the classic pattern, which can be diagnosed at an early stage and has little lymph node involvement; usually it has a higher survival rate than IBC and can be cured with surgical excision. It may develop lung recurrences and metastasis; however, it still has a high survival rate [3,26]. Mucoepidermoid carcinoma has more similarities than differences to its salivary gland counterpart and low-grade tumors have a good postoperative prognosis. Polymorphic carcinoma being slows growing and locally aggressive with some metastatic potential; nevertheless, it tends to have a better prognosis in comparison with other TNBCs [3,27]. Tall cell carcinoma with reversed polarity shows a painless clinical course, with very low proliferative indexes (<5%), rare axillary lymph node metastasis and good postoperative prognosis. Most of the adenomyoepitheliomas are benign and surgery is curative [28].
Essentially, TNBCs are treated with conventional neoadjuvant chemotherapy. However, there seems to be a lack of biological reasons that justify such a decision and the impact on prognosis is not clear [17]. Overall, there are good response rates to conventional neoadjuvant chemotherapy which are directly associated with the proliferative index shown by the neoplasm: high proliferative indexes are associated with a better response to neoadjuvant therapy, in comparison with those with low proliferative indexes [29]. Although there is not enough evidence in some subtypes, it seems reasonable to consider low-grade TNBC as a group with characteristic genetic alterations and low response rates to conventional chemotherapy, partly due to low proliferative indexes (defined as TNBC with proliferative indexes <30%) Furthermore, the susceptibility to radiotherapeutics treatment of some lowgrade TNBC, such as the metaplastic subtype, is well documented [1,3,13]. It should be noted that low grade TNBC could not express any tumor markers of mammary origin. Despite recent progress, some tumors of metastatic presentation with a primary of unknown origin may fall within this profile, so a high degree of suspicion and a good radiological correlation would be necessary for their correct diagnosis [30].
We emphasize that low-grade TNBC is just a mere descriptive term, but it could be used as a diagnostic term in a CNB if a specific subtype may not be established, referring the definitive diagnosis to the study of the surgical specimen. A correct diagnosis of a specific histopathological subtype from CNB is possible in certain cases and this allows adequate treatment within a multidisciplinary breast unit in an increasingly frequent, neoadjuvant regimens context. Although conducting additional IHC may delay the diagnosis, it is essential to distinguish clearly between low-grade TNBC and other TNBC, as they have a different prognosis and require different therapeutic management during its evolution as a disease.
Citation: Martin JR. Essentials on Low-grade Triple-negative Breast Cancer. Med Rep Case Stud, 2021, 06(6), 225
Received: 13-Sep-2021 Published: 04-Oct-2021, DOI: 10.35248/2572-5130.21.6.225
Copyright: © 2021 Martin JR. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : NO