Research Article - (2021) Volume 12, Issue 11
Parkinson's disease (PD) is a progressive neurodegenerative disorder that affects nerve cells, or neurons, in the part of the brain that controls movement. A hallmark feature of PD is the degeneration of the dopamine neurons in the substantia nigra (SN) pars compacta and the consequent striatal dopamine deficiency. Yet, the pathogenesis of PD remains unclear.The lack of dopamine causes the primary symptoms of Parkinson's disease - tremor, slowness of movement, muscle stiffness and balance problems. In vivo animal models have simulated most, although not all, of the hallmarks of PD and are useful for testing new neuroprotective approaches. Research is devoted to the study of systemic compensatory reactions of the rat's brain developing in response to rotenone-induced animal model of PD under the conditions of neuroprotective intervention of Curcumin. This has raising expectations for the development of new neuroprotective therapies for the prevention of PD. Male albino rats were treated with rotenone injections (2.5 mg/ml intraperitoneally) for 21 days. We examined the effects of neuroprotector curcumin (200 mg/kg) on behavior and the electrical activity of hippocampus neurons measured in response to high frequency stimulation (HFS) of entorhinal cortex (EC). In the hippocampus, the excitatory and inhibitory synapses between EC and CA3 pyramidal cells expresses robust forms of short-term plasticity, such as frequency facilitation (post-tetanic potentiation-PTP) and depression (post-tetanic depression- PTD). Motor activity was assessed by cylinder test. The results showed that Rotenone causes significant reduction of neuronal activity, whereas curcumin can improve the motor impairments and electrophysiological parameters and may be beneficial in the treatment of PD. Curcumin significantly prevented rotenone-induced impairment of hippocampal synaptic plasticity, which is likely mediated via dysfunction of mitochondrial complex I. It alleviated the deficits behavior in rats as the rearing frequencies of animals were enhanced.
Parkinson’s disease • Hippocampus • Entorhinal cortex • Rotenone • Curcumin • Cylinder test
Parkinson's disease (PD) is the most common neurodegenerative disease of unknown origin. It is characterized by motor symptoms: resting tremor, muscle rigidity, bradykinesia, lack of postural reflexes, as well as complex interaction of multisystem degeneration and depletion of neurotransmitters, loss of dopaminergic neurons in SN as well as in other parts of central nervous system [1]. Cholinergic denervation occurs in the early stages of PD [2].
Over the past decade, researchers have become more interested in the neuroprotective effect, which has led to numerous publications about neuroprotective and disease-altering agents. This has raised expectations for the development of new neuroprotective therapies for the prevention of PD, but to date nobody could achieve it [3].
Curcuma rhizome, belonging to the Zingiberaceae family, is the root of Curcuma longa plant. Curcuma has a long history of use in traditional medicine of China and India, and it is used as a curry spice in food preparation [4]. Of the three main Curcuminoids found in the turmeric root, Curcumin is the most abundant and the most biologically active. Curcumin (C21H20O6) is the main pharmacologically active ingredient of the Curcuma rhizome. It is safe, with little to no toxicity, and possesses antitumor [5], antioxidant [6,7], antiinflammatory [8,9], antiapoptosis, and lipid-reducing pharmacological effects [10]. As an anticarcinogen, an important target of Curcumin is the Keap1 protein, which normally binds and sequesters Nrf2 in the cytoplasm. Curcumin can directly act on Keap1 to release Nrf2, which then translocates to the nucleus, where it heterodimerizes with small Maf proteins and binds to antioxidant response elements, inducing the expression of a large number of cytoprotective genes [11]. It was shown that Curcumin can attenuate diabetes induced apoptosis in retinal neurons by reducing the glutamate level and down regulating calcium/calmodulin-dependent protein kinase I [12]. There are literature data stating that Curcumin significantly prevented the stress-induced decrease in 5-HT (1A) mRNA and BDNF (brain-derived neurotrophic factor) protein levels in the hippocampal subfields, two molecules involved in hippocampal neurogenesis [13]. Recent studies have shown that Curcumin inhibits β-secretase and acetylcholinesterase in Aβ-induced animal model of Alzheimer's disease [14,15]. Curcumin has been reported to inhibit the activity of a variety of signaling enzymes in cells, such as NF-κB, mitogen-activated protein kinases, cyclooxygenase-1, Bcl-2, Bcl-xL and cyclin D1, that contribute to cellular survival and proliferation [16]. In Parkinson's disease rat model Curcumin increased the contents of monoaminergic neurotransmitters, such as dopamine and norepinephrine [17].
Animal studies, in which researchers simulated depression by exercising animals to the point of exhaustion, have indicated that these remedies may work. They have shown that Curcumin inhibits the enzyme monoamine oxidase in the brain. Monoamine oxidase neutralises neurotransmitters, and administration of Curcumin therefore boosts the concentration of serotonin, dopamine and noradrenalin in the brain. The neurochemical assays have shown that Curcumin produces a marked increase of serotonin and noradrenaline levels at 10 mg/kg in both the frontal cortex and hippocampus [18].
In the animal study it was shown that doses of 10 and 20 mg Curcumin per kg per day resulted in hippocampus growth by boosting the synthesis of the neural growth hormone BDNF [19]. An increase in BDNF synthesis is the result of the enzyme extracellular signal-related kinase being activated in brain cells. Curcumin has been shown to have the ability to block or reverse the stress-induced changes typical of hypothalamic– pituitary–adrenal axis dysfunction to a level comparable to a typical tricyclic antidepressant including increases in corticosterone levels, reduced glucocorticoid receptor mRNA expression, and reduced levels of phosphorylated CREB (cAMP response element-binding protein).
Rotenon is a pesticide, which causes behavioral, biochemical and morphological changes in rats and reproduces features of Parkinson's disease, including selective nigrostriatal dopaminergic degeneration. Rotenone was evaluated in 2000 for mitochondrial complex I-1-methyl-4- phenyl-1,2,3,6-tetrahydropyridine (MPTP)-like inhibition [20]. Like MPTP, rotenone also easily crosses the blood-brain barrier, where it causes oxidative stress and accumulates in dopaminergic neurons. However, reducing ATP is not a cause of cell death. Moreover, the large number of reactive oxygen species causes damage in substantia nigra (SN) [21,22]. According to some researchers, a possible advantage of the Rotenone model is that it accumulates the protein alpha synuclein, which is one of the key determinants of PD, while MPTP and 6-hydroxidophamine don’t cause such accumulations. In addition, some rats are internally resistant to rotenone, with only about 50% developing neurodegeneration. Rotenone experiments require a large number of animals [23]. This method is not preferred by many researchers, although it induces key PD features that do not produce MPTP or 6-hydroxidophamine.
Previous studies have shown that intravenous injection of rotenone induces dopamine deficiency and L-DOPA-responsible locomotor disorders [24,25]. Rotenone injection (daily, intraperitoneal) causes dopamine depletion of the striatum, degeneration of the dopamine terminals of the striatum, and loss of tyrosine hydroxylase-positive neurons in SN [26].
Comparing intravenous-subcutaneous injections of rotenone with osmotic pumps with the intra-abdominal method, it has been shown that the intra-abdominal method causes the accumulation of alpha-synuclein protein aggregation in SN [27]. Activation of astrocytes and microglial cells is also involved in rotenone-induced neurotoxicity, which is another characteristic of PD [28].
In the course of the current study the modulatory effects of Curcumin on rats’ hippocampal neuronal activity have been demonstrated under high frequency stimulation (HFS) of ipsilateral EC as well as behavioral and morphological features concened.
Experimental procedures
All experiments were conducted using protocols approved by the Committee of Ethics of the Yerevan State Medical University (YSMU) (Yerevan, Armenia). All animal procedures were carried out in accordance with the European Communities Council Directive 2010/63/UE and the local Animal Care Committee. Adult male albino rats (n=20) weighing 200±20 g were purchased from the experimental center of Orbeli Institute of Physiology. The animals were maintained at 25±2°C and 12 h light– dark cycle, lights on 07:00–19:00 h. The animals were provided food and water ad libitum. Rotenone was dissolved in sunflower oil (2.5 mg/ml) and was injected intraperitoneally (IP) (1ml/kg 3 weeks). Control group rats were injected sunflower oil (1 ml/kg). Dimethyl sulfoxide (DMSO) (1ml/kg) was injected daily IP (3weeks). Curcumin (200 mg/kg IP) was dissolved in dimethyl sulfoxide (DMSO, 1 ml/kg).
The animals were divided randomly into 4 groups (n = 6): rotenone (n=6), sunflower oil (n=6), dimethyl sulfoxide (DMSO)(n=6),or Curcumin (n=6).
R– Rotenone group, 2.5 mg/kg (IP) for 3 weeks
SO- Sunflower oil group, 1 ml/kg (IP) for 3 weeks
RD-Rotenone (3 weeks) + DMSO, 1ml/kg (IP) group for 3 weeks
RC- Rotenone (3 weeks) + Curcumin group, 200 mg/kg (IP) for 3 weeks
N-norm group
In groups RD and RC Curcumin and DMSO were administered 3 weeks after PD induction. Animals were studied after 6 weeks and in vivo electrophysiological analysis was done.
Chemicals
Curcumin and rotenone were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO, USA). Other chemicals were provided by local commercial sources.
Electrophysiology and data analysis
In acute in-vivo experiment the animals were anesthetized (Urethan 1.2 g/kg), immobilized with 1% ditiline (25 mg/kg i/p), fixed in a stereotaxic head frame and were transferred to artificial respiration. The sample of isolated rat brain was obtained by transection of spinal cord (T2–T3). The stimulating electrode were inserted into the ipsilateral EC according to stereotaxic coordinates [29] (AP–9, L ±3.5, DV+4.0 mm) and a glass recording electrode (1–2 μm tip diameter) filled with 2 M NaCl was inserted into the hippocampal field at coordinates (AP−3.2-3.5; L±1.5-3.5; DV +2.8–4.0 mm) for recording spike activity flow of single neurons. HFS (100 Hz during 1 sec) was performed by means of rectangle pulses of 0.05 ms duration and 0.08-0.16 mA amplitude. Recording and mathematical analysis of spike activity were carried out on the basis of the special program providing selection of spikes by amplitude discrimination, which pinpoints spikes and excludes artifacts during HFS, allowing not only post- tetanic, but also tetanic activity evaluation [30].
Behavioral study and cylinder test
The effects of Curcumin on the rat behavior were studied by cylinder test. Studies were begun 24 h after the injections. The animals were placed in a clear Plexiglas cylinder (20 cm in diameter and 30 cm in height) in order to evaluate motor asymmetry. A mirror was placed to the side of the cylinder at an angle to enable the recording of forelimb movements even when the animal was turned away from the camera. Scoring was done by an experimenter blinded to the condition of the animal using a video cassette recorder with slow-motion and clear stop-frame capabilities. A video camera above the field was connected to a video recorder and a monitor, recording the movement of the rat. During rearing, behavior, the forelimbs will contact the wall of the cylinder. The apparatus was cleaned with 5% ethanol solution before behavioral testing to eliminate possible bias due to odors left by previous rat. Rats were tested only once to prevent habituation to the apparatus. To be classified as a rear, the animal had to raise forelimbs above shoulder level and make contact with the cylinder wall with either one or both forelimbs. Removal of both forelimbs from the cylinder wall and contact with the table surface was required before another rear was scored. Forelimb contacts while rearing are scored with a total of 5 min for each animal. Data of all the results were presented as mean± SEM. Significant differences between groups were calculated using Student’s t-test and p< 0.05 was considered statistically significant. Cylinder test parameters were studied on daily basis.
Morphological study
After each electrophysiological experiment, animals with a three-week PD model were sacrificed; the brain was fixed in 10% formalin solution. Serial frozen midbrain sections were stained with 1% methylene green solution or Nissl toluidine blue. Nissl staining method is one of the main histological methods and widely used in modern research to detect both structural and functional state of neurons, as well as to assess the normal state and pathological changes of nerve cells. A rat brain atlas was used for the analysis of histological preparations.
Types of HFS-Induced reactions
An electrophysiological analysis in Norm group (264 neuron) (Figure 1), showed that the tetanic potentiation TP and PTP during HFS (100 Hz) in the hippocampus expressed 4.12 times (53.96:13.10), TP PTD expressed 2.71 times (28.39:10.47). The proportion of hippocampal neurons with TP responses predominates (21.97%) (Table 1) (Figure 1).
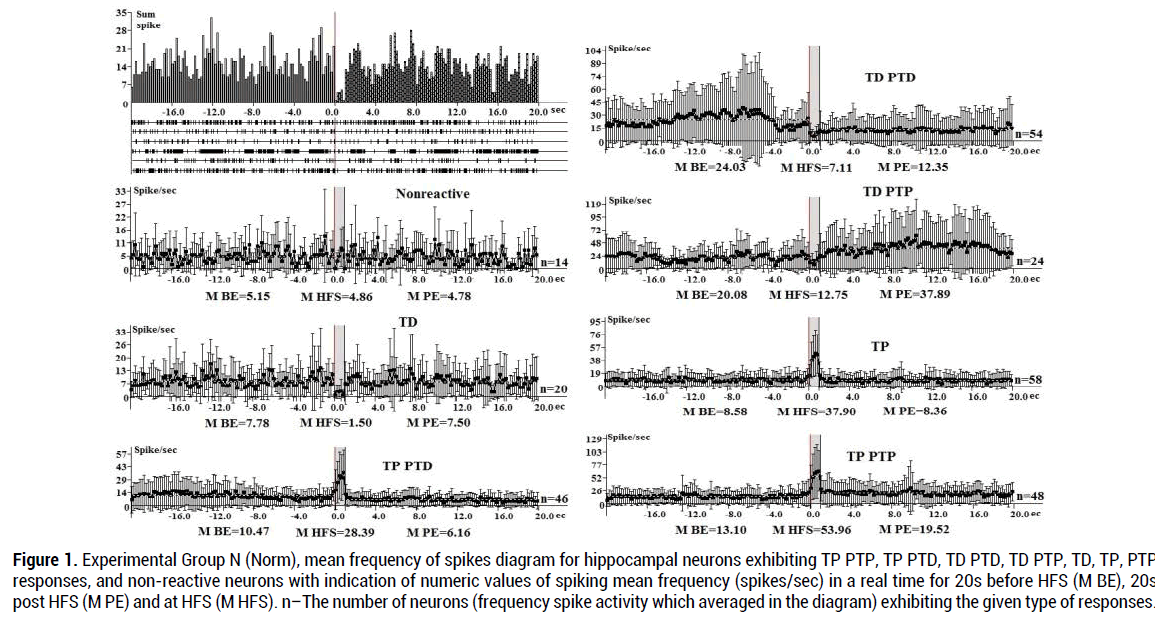
Figure 1. Experimental Group N (Norm), mean frequency of spikes diagram for hippocampal neurons exhibiting TP PTP, TP PTD, TD PTD, TD PTP, TD, TP, PTP responses, and non-reactive neurons with indication of numeric values of spiking mean frequency (spikes/sec) in a real time for 20s before HFS (M BE), 20s post HFS (M PE) and at HFS (M HFS). n–The number of neurons (frequency spike activity which averaged in the diagram) exhibiting the given type of responses.
| Response type | Percentage of response types | M BE spike/sec |
M HFS spike/sec |
M PE spike/sec |
|---|---|---|---|---|
| TP-PTP (4.12 times) | 18.18 % | 13.10 | 53.96 | 19.52 |
| TP(4.41 times ) | 21.97 % | 8.58 | 37.90 | 8.36 |
| TP PTD(2.71 times ) | 17.42 % | 10.47 | 28.39 | 6.16 |
| TD PTD(3.38 times ) | 20.45 % | 24.03 | 7.11 | 12.35 |
| TD PTP(1.58 times ) | 9.09 % | 20.08 | 12.75 | 37.89 |
| Non-reactive | 5.3 % | 5.15 | 4.86 | 4.78 |
| TD(5.2 times ) | 7.58 % | 7.78 | 1.50 | 7.50 |
Table 1. Pre-stimulation (M BE), post-stimulation (M PE) and high frequency stimulation (M HFS) percentage of spike activity and mean frequency values (spike/sec) in the experimental control group in hippocampal neurons
During HFS tetanic depression with TD PTD responses expressed 3.38 times (24.03:7.11), TD PTP expressed 1.58 times (20.08:12.75) (Figure 1), TD- 5.2 times (7.78:1.5), non-reactive- 5.3%
An electrophysiological analysis in Group R (189 neurons) showed that the tetanic potentiation during HFS (100 Hz) in the hippocampus with TP PTP expressed 1.7 times (15.76:9.36), TP PTD responses 1.86 times. (Figure 2) Inhibitory (tetanic depression) during HFS (100 Hz) of EC in neurons with TD PTD and TD PTP responses expressed respectively by 1.69 (10.70:6.35) and 2.06 (11.71:5.68) times. PTP responses- 1.39 times, TP (6.34%), TD-1.72 times (Figure 2), non-reacitive- 5.34%: The proportion of TD PTP and TP PTP responses in hippocampal neurons is predominant (respectively 20.1%, Table 2).
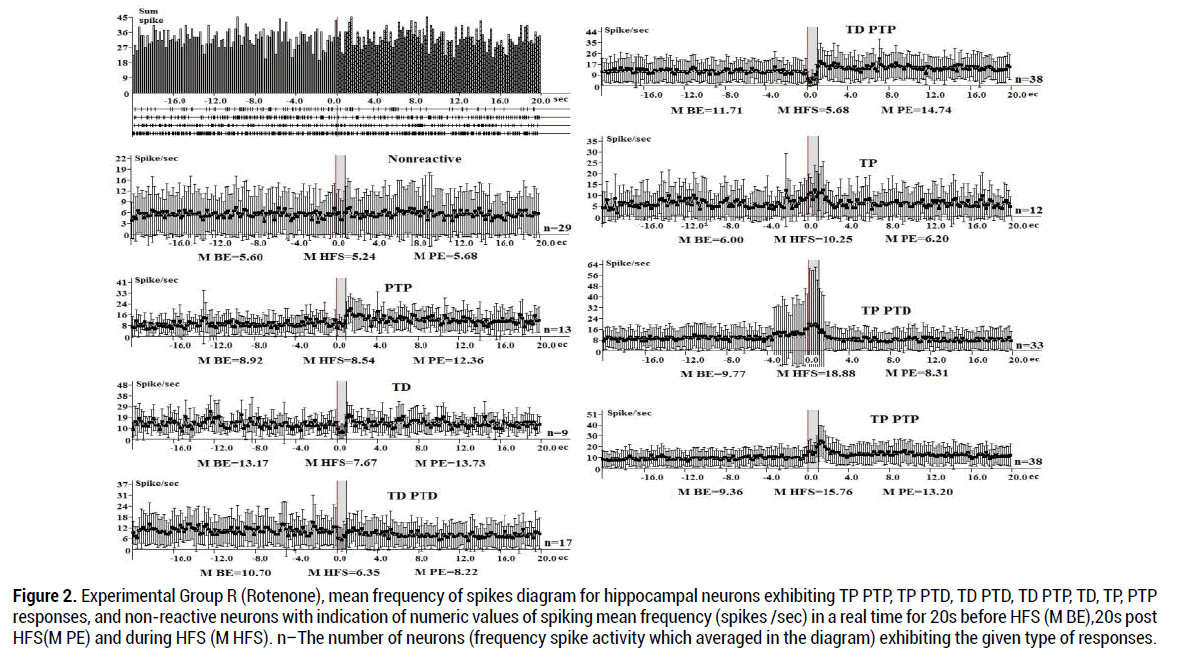
Figure 2. Experimental Group R (Rotenone), mean frequency of spikes diagram for hippocampal neurons exhibiting TP PTP, TP PTD, TD PTD, TD PTP, TD, TP, PTP responses, and non-reactive neurons with indication of numeric values of spiking mean frequency (spikes /sec) in a real time for 20s before HFS (M BE),20s post HFS(M PE) and during HFS (M HFS). n–The number of neurons (frequency spike activity which averaged in the diagram) exhibiting the given type of responses.
| Response type | Percentage of response types | M BE spike/sec |
M HFS spike/sec |
M PE spike/sec |
|---|---|---|---|---|
| TP PTP (1.7 times) | 20.1 % | 9.36 | 15.76 | 13.20 |
| TP PTD (1.86 times ) | 17.46 % | 9.77 | 18.18 | 8.31 |
| TD PTD(1.69 times ) | 8.99 % | 10.70 | 6.35 | 8.22 |
| TD PTP(2.06 times ) | 20.1 % | 11.71 | 5.68 | 14.74 |
| TP(1.7 times ) | 6.34 % | 6.00 | 10.25 | 6.20 |
| Non-reactive | 15.34 % | 5.60 | 5.24 | 5.68 |
| PTP(1.39 times ) | 6.87 % | 8.92 | 8.54 | 12.36 |
| TD(1.72 times ) | 4.76 % | 13.17 | 7.67 | 13.73 |
Table 2. Hippocampal neurons pre-stimulation (M BE), post-stimulation (M PE) and high frequency stimulation (M HFS) percentage of spike activity and mean frequency values (spike/sec) in Rotenone group.
In the SO group (338 neurons) tetanic potentiation during HFS in hippocampal neurons with TP expressed 1.38times (28.18:20.8 spike/s), tetanic depression during HFS in neurons with TD PTD responses–3.64 times (27.08:7.44 spike/s), TD PTP responses – 4.22 times (21.94: 5.19), TD-4.66 times (22.65:4.86). In general, during HFS (M TT) in the SO group the TP responses show the lowest share (3.55%) (Figure 3).
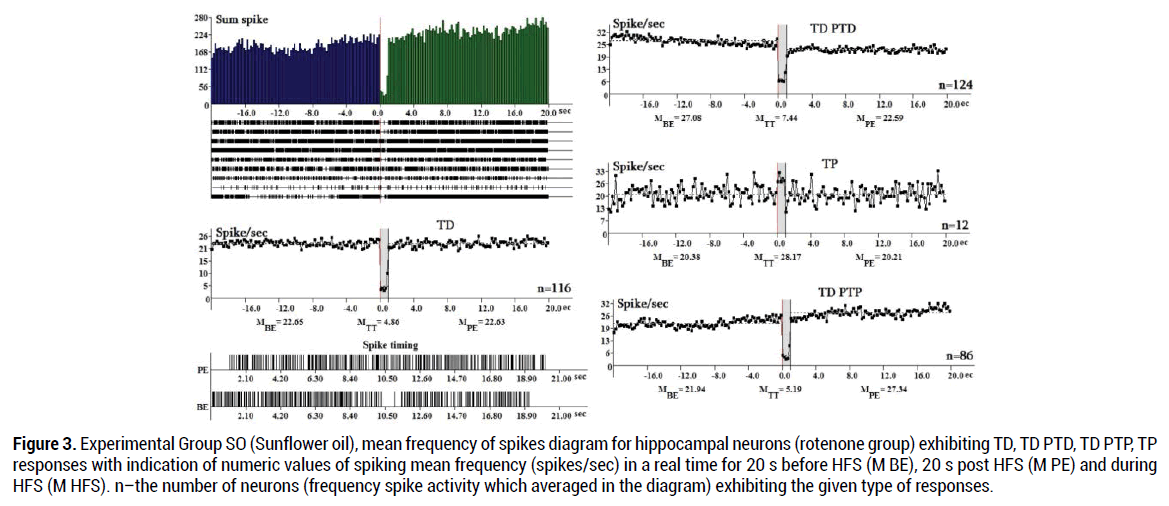
Figure 3. Experimental Group SO (Sunflower oil), mean frequency of spikes diagram for hippocampal neurons (rotenone group) exhibiting TD, TD PTD, TD PTP, TP responses with indication of numeric values of spiking mean frequency (spikes/sec) in a real time for 20 s before HFS (M BE), 20 s post HFS (M PE) and during HFS (M HFS). n–the number of neurons (frequency spike activity which averaged in the diagram) exhibiting the given type of responses.
In the RD group (272neurons) tetanic potentiation during HFS in hippocampal neurons with TP PTP expressed 1.52 times (19.75:12.98 spike/s), tetanic depression during HFS in neurons with TD PTD responses–3.9 times (33.29:8.52 spike/s), TD PTP responses– 3.72 times (34.66:9.32), TD-3.59 times (39.67:11.04). In general, during HFS (M HFS) in the RD group the TD PTP responses show the highest share (60.3%) (Figure 4).
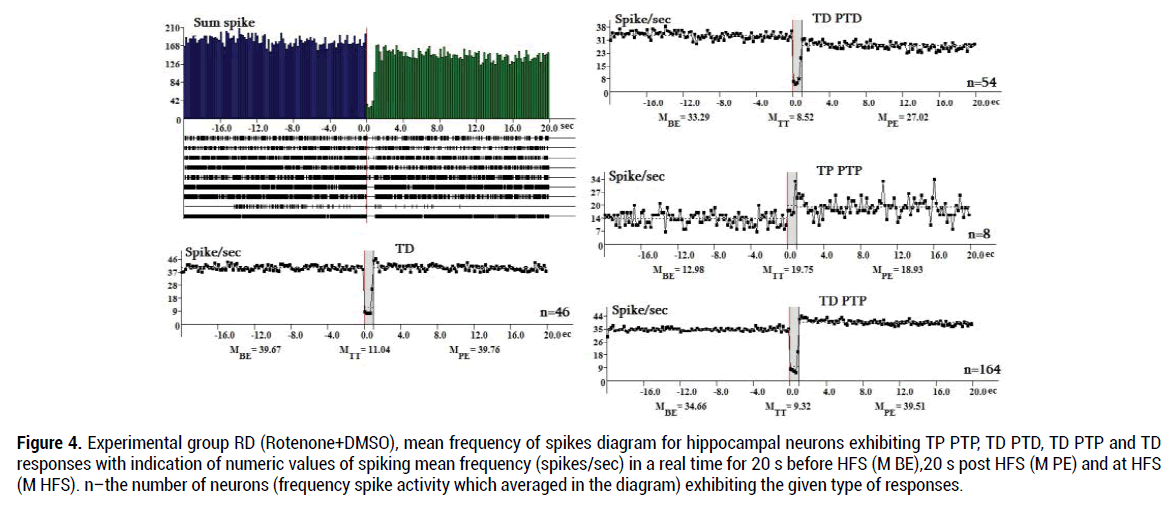
Figure 4. Experimental group RD (Rotenone+DMSO), mean frequency of spikes diagram for hippocampal neurons exhibiting TP PTP, TD PTD, TD PTP and TD responses with indication of numeric values of spiking mean frequency (spikes/sec) in a real time for 20 s before HFS (M BE),20 s post HFS (M PE) and at HFS (M HFS). n–the number of neurons (frequency spike activity which averaged in the diagram) exhibiting the given type of responses.
In the RC group (Figure 5) tetanic potentiation during HFS in hippocampal neurons with TP expressed 1.59 times (МTT=19.75 / MBE=12.41 spike/s), tetanic depression during HFS in neurons with TD PTD responses – 4.49 times (MBE = 35.23 / МTT = 7.84 spike/s), TD PTP responses – 3.28 times (MBE = 37.55 /МTT = 11.45), TP PTD responses 2.89times (МTT=115.14/MBE=39.78),TD-4.08 times (MBE=30.48/МTT =7.48). Ingeneral,duringHFS(MHFS) in the RC group the TP and TP PTD responses show the lowest share (2.4% and 4.2%). TD PTD responses show the highest share (47.3%,).
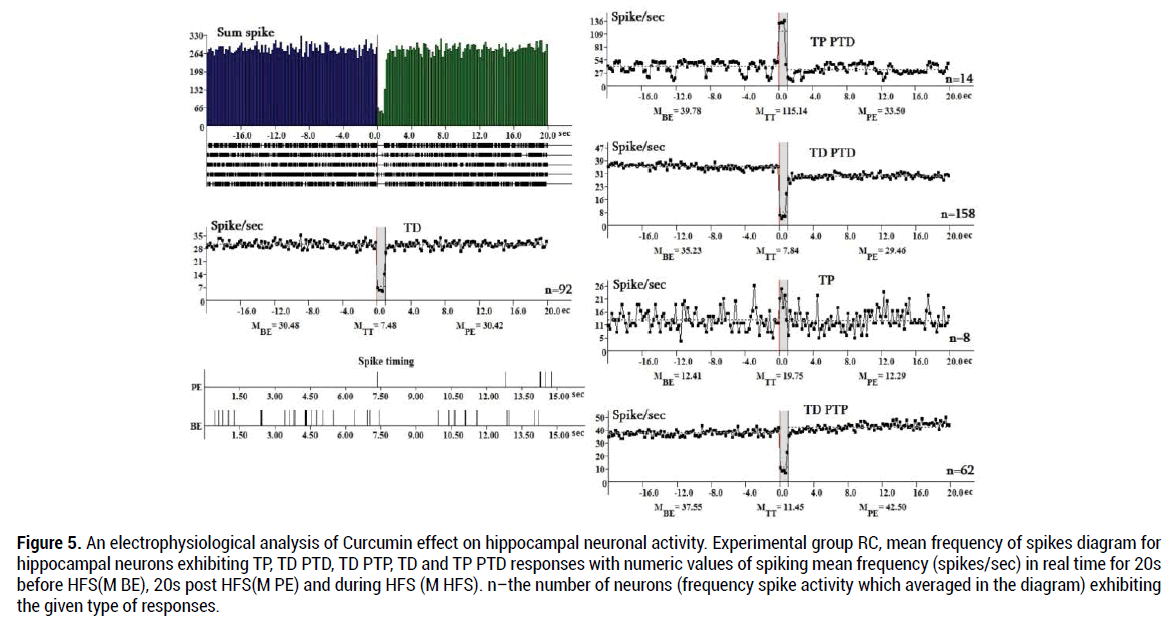
Figure 5. An electrophysiological analysis of Curcumin effect on hippocampal neuronal activity. Experimental group RC, mean frequency of spikes diagram for hippocampal neurons exhibiting TP, TD PTD, TD PTP, TD and TP PTD responses with numeric values of spiking mean frequency (spikes/sec) in real time for 20s before HFS(M BE), 20s post HFS(M PE) and during HFS (M HFS). n–the number of neurons (frequency spike activity which averaged in the diagram) exhibiting the given type of responses.
Tetanic potentiation with TP PTP responses appeared in the groups R and RD. Tetanic depression in neurons with TD PTD responses also more expressed in the group R (5.02 times) in comparison with the group RC (4.49 times) (Figure 5).
Morphological analysis of changes in the hippocampus
In addition to electrophysiological investigations, morphological studies of the hippocampus were carried out in order to identify pathological changes caused by rotenone and recovery features of Curcumin. Following introduction of rotenone and Curcumin the material was taken at the end of each electrophysiological experiment.
Hippocampal neurons have varying degrees of stability against rotenoneinduced intoxication following Curcumin treatment. The pyramidal cells are hyperchromic, the chromatin is unevenly distributed in them and often has a peripheral position. However, some neurons have a light-stained nucleus located in the center or in the periphery. Axons are straight, the outlines of the cells are clear. Such neurons are able to survive because pathological changes are reversible. Some cells are arranged in pairs or groups, which is typical for hippocampal cells under conditions of adverse effects. It should be noted that normal hippocampal neurons differ from other structures by chromatophilia and intense staining, and it does not always depend on the diagnosed change.The morphological picture completely corresponds to the norm (Figure 6).
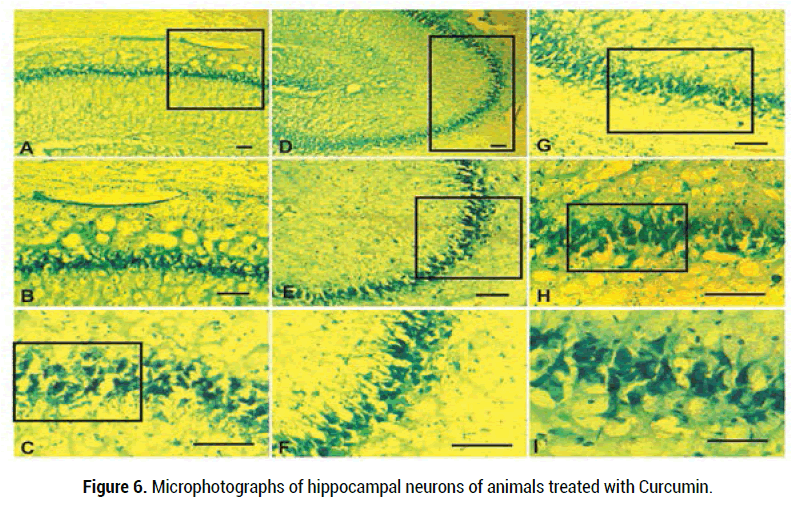
Figure 6. Microphotographs of hippocampal neurons of animals treated with Curcumin.
Scale: A-H- 100 μm, I-50 μm
Curcumin significantly prevented rotenone-induced impairment of hippocampal synaptic plasticity, which is likely mediated via dysfunction of mitochondrial complex I. It was shown that Curcumin is able to protect hippocampal neurons against NMDA-induced cell death, confirming its antiexcitotoxic property and induced an increase in NMDAr subunit type2A (NR2A) level, with kinetics closely correlated to time-course of neuroprotection and decrease in [Ca(2+)](i) [31]. Curcumin’s protective effects appear to be mediated by inhibition of apoptosis, as the rewasha dose dependent reduction in apoptosis and a parallel decrease in caspase3 levels [32]. Curcumin attenuated Aβ-induced elevation of the ratio of cellular glutamate/γ-aminobutyric acid (GABA) with a concentrationdependent manner [33]. It was shown that chronic Curcumin treatment resulted in increased BDNF expression in the hippocampus. BDNF has been directly implicated in cell survival and neurogenesis [34]. Curcumin interaction with various neurotransmitters, e.g., serotonin and dopamine [35], GABAergic system, and glutamatergic system have been reported. In-vitro studies indicate a protective effect of Curcumin against NMDAmediated toxicity, suggesting inhibitory effects of Curcumin on NMDA receptors. It was found that Curcumin protects hippocampal neurons against NMDA-induced apoptosis [36]. The effects of Curcumin also have been tested in rotenone based cell and Drosophila PD models. Curcumin also rescued rotenone-induced locomotor impairment and early mortality and restrained dopaminergic neuronal degeneration in Drosophila via reducing mitochondrial ROS levels [37]. Previous studies have shown the cytoprotection of Curcumin against 6hydroxydopamine (6-OHDA)- induced neuronal death and the neuroprotective effects of Curcumin were attributed to the modulation of nuclear factor-kappaB translocation [38]. Thus, Curcumin protects hippocampal neurons against rotenone-induced cell death.
Behavioral study
In the Parkinson rat, there is a decreased reliance on the impaired forelimb for movements involving a response to weight shift.The animals preferentially initiate movement with the nonimpaired forelimb, particularly for lateral movements during vertical exploration of surfaces. Although PD is characterized by movement disorders in later stages of the disease, it is now appreciated that there also may be cognitive impairment, including dementia and behavioral changes [39]. Behavioral assessments were carried out before the start of the treatment, then regularly at an interval of 42 days post treatment and final behavioral quantification was done after 24 h of last dose.
Four groups of Rotenone and sunflower oil were tested. In this study, both forelimbs were averaged together because rotenone produces bilateral symptoms. As rotenone affects movement behavior bilaterally, activity was measured by counting the number of rearings made by each animal in 3 and 5-min period without the use of specific limbs recording. 24 h after the last day of treatment, the rats were evaluated with behavioral test. The cylinder test was performed according to the method described previously [40]. In the present study, the rotenone dose (2.5 mg/kg/day) for 21 days caused significant behavioral changes. No Parkinson's-like symptoms were reported in sunflower oil (1 ml/kg) group. In each period, 3 tests were recorded for each forelimb, and were averaged (Figures 7,8).
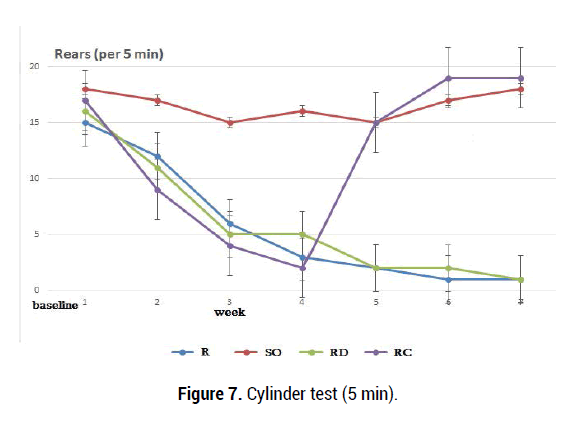
Figure 7. Cylinder test (5 min).
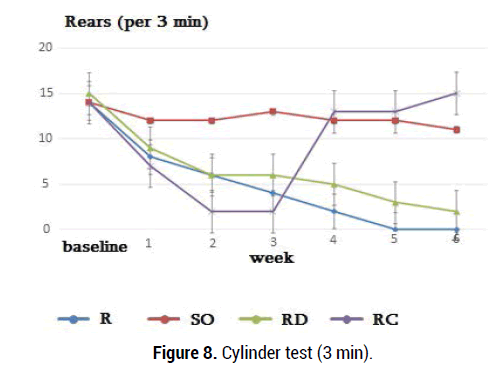
Figure 8.Cylinder test (3 min).
Spontaneous rearings were calculated for animals receiving sunflower oil (1 ml/kg), DMSO, Curcumin and rotenone (2.5 mg/kg/ day), which were tested before injection and 1-6 weeks after injection Any animal that showed severe signs of illness during the experiment was prematurely euthanized. All animals lived throughout the experiment. The forelimb deficit is quantified using the front paw unit (0 indicates that the front paw function is missing and 20 indicates that there is no deficit). Rotenone rats showed slightly worse indices than control rats but this difference was statistically significant (p<0.05)but, this difference is statistically significant (p<0.05). The control rats were compared with rats receiving rotenone (3 weeks later) and motor changes were found. Postural instability is at the root of a number of behavioral deficits that have been considered common in animal models of PD. Animals are more likely to initiate movement with an uninjured front paw, particularly during examination of surfaces on vertical lateral movements [41] (Figure 9).
Figure 9. Sequence and time of touching the forelimbs on the wall of the cylinder.
The anti-apoptotic properties of Curcumin have been reported in neurodegenerative diseases and it has been shown that Curcumin improves neuropathological changes [42]. We found that rats receiving rotenone showed a stable decrease in standing for 3 weeks compared with animals receiving sunflower oil. Rotenone (2.5 mg/kg ip) administration throughout the test causes a stable standing deficit. Rats receiving rotenone injection during a 5-minute behavioral test (3-6 weeks) showed abrupt behavioral changes than rats in group II and this difference is statistically reliable (p = 0.0001, p <0.05). Behavioral indices of R and SO groups of rats were compared with those of rats receiving Curcumine (3 weeks later, group 4) and significant changes were observed (p=0.0003, p<0.05). Observation of 3-minute movements in rats receiving rotenone (3-6 weeks) resulted in abrupt behavioral changes (Figure 8) compared with the SO group rats and this difference is statistically significant (p=0.0001, p<0.05). When behavioral indices of R and RD groups of rats were compared with those of rats receiving Curcumin (3 weeks later, 200 mg /kg, group RC) a reliable positive changes were observed (p=0.0002, p=0.0007 p<0.05). After curucumin treatment, the rearing and postural disorders observed in the R and RD groups were restored. The results of tubular test in the group RC were almost completely restored at 4, 5, 6 weeks after rotenone administration and were close to the initial values of group SO and N (norm group).
Experimental animals were injected with rotenone for 21 days to assess the state of neuronal cells in the hippocampus. These data were compared with the results obtained in control animals. During electrophysiological study of the hippocampus of control and experimental animals, changes in neuronal activity have been observed in both groups. The rotenone model is one of the toxic models of PD, which causes nigrostriatal degeneration in rodents and induces PDlike symptoms. The rotenone rat model has gained some reliability as a pesticide model of PD and reproduces most of the symptoms of movement disorders and histopathological features of PD (for example, the formation of Lewy bodies) [43]. In the pathogenesis of PD, mitochondrial dysfunction, oxidative stress, excitotoxicity, dysfunction of the ubiquitin-proteasome pathway, and apoptosis have been identified. The major mechanism of PD pathogenesis is the death of dopaminergic neurons in the midbrain, induced by mitochondrial dysfunction, oxidative stress and neuroinflammation, which results in activation of microglia, cytotoxicity of some metabolites, increased functional activity of cells, and impaired neuroglial interactions [44]. The hallmark pathology of PD includes the loss of dopaminergic neurons in the substantia nigra pars compacta (SNc) and pathological alpha-synuclein (aSyn) aggregation in Lewy bodies/Lewy neurites [45]. While not yet widely appreciated there is growing evidence that the dopaminergic neurons of the ventral tegmental area (VTA) are also damaged in PD [46].
In rats, NMDA receptors are highly expressed in hippocampal CA1 region. The CA1 field of the hippocampus is also characterized by high density of other excitatory amino acid receptors, such as metabotropic mGluR1 receptors [47]. Pyramidal and granular neurons make up 90% of the total number of neurons in the hippocampus. The remaining 10% are represented by GABAergic interneurons [48]. Interneurons form an organized network that controls and regulates the functioning of pyramidal and granular neurons. GABAergic interneurons receive GABAergic innervation from extra-hippocampal regions of the brain, while other groups of interneurons send axonal projections to other extrahippocampal regions of the brain. The hippocampal region contains many noradrenergic, serotonergic, and cholinergic axonal terminals [49].
It has recently been shown that dopamine has an excitatory effect only on some intracaudate neurons, which receive direct excitatory influences from SN. In response to SN stimulation, about 50% of neurons are inhibited, and only about 15% of cells are excited [50]. Irreversible rotenone interference with glutamatergic transmission may result from a deficiency in glutamate transport to presynaptic terminals. Thus, rotenone can reduce the level of presynaptic glutamate release. Moreover, the inhibitory effect of rotenone on neuronal responses may be at the presynaptic rather than postsynaptic level [51]. It has been reported that rotenone reduces field potentials (fEPSP) by 17% in the CA1 region of the hippocampus. There are other reports of an increase in NMDA responses by rotenone in rat midbrain slices, and this potentiating effect is associated with the activation of NMDA receptors [52]. NMDA receptors allow membrane transport of sodium, potassium and calcium ions to work as coincidence detectors, from the moment their channels open, requiring depolarization and release of glutamate from presynaptic neurons simultaneously. Kainate and AMPA receptors allow membrane transport of sodium and potassium ions [53].
Our experimental results show that rotenone has an inhibitory effect on synaptic transmission in hippocampal neurons. The inhibitory effects are presumably the result of an indirect influence switched through GABAergic cells. After curcumin treatment, the rearing and postural disorders mainly were restored. The tubular test testified that in general motor patterns almost completely restors at 4, 5, 6 weeks after curcmin administration and were close to the initial normal values.
The authors declare no conflict of interest.
The authors thank Mr. V.S Kamenetski, Orbeli Institute of Physiology, NAS RA for development of the spetial program providing selection of spikes by amplitude discrimination and Dr.S.Badalyan for assistance with morphological data interpretation.
LVD and VHS conceived of the project and conducted pilot studies; LEH and LPM extended analyses with KVS and NVS; LVD did rat behavioral study and morphology with LEH, LPM and NVS; VHS and LVD analyzed data and wrote the paper with editorial assistance from KVS.
The sponsors did not play any role in study design; collection, analysis and interpretation of data; writing of the report; or decision where to submit the article for publication.
Abbreviations: PD, Parkinson's disease; EC, entorhinal cortex; BDNF, brain-derived neurotrophic factor; CREB, cAMP response elementbinding protein; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; DMSO, Dimethyl sulfoxide; IP, intraperitoneally; HFS, high frequency stimulation; TP,TD, Tetanic potentiation and depression; PTP,PTD, post tetanic potentiation and depression; R, Rotenone; SO, Sunflower oil; RD, Rotenone +DMSO; RC, Rotenone + Curcumin; N, norm; SN, substancia nigra; M BE, mean frequency before event; M PE, mean frequency post event; M HFS, mean frequency during HFS; VTA, ventral tegmental area; ATP, adenosine triphosphate; L-DOPA, Levodopa.
Citation: Mbamalu ON, Antunes E, Silosini N, Samsodien H, Syce J (2016) HPLC Determination of Selected Flavonoid Glycosides and their Corresponding Aglycones in Sutherlandia frutescens Materials. Med Aromat Plants 5:246. doi:10.4172/2167-0412.1000246
Received: 13-Oct-2021 Published: 15-Nov-2021
Copyright: © 2021 Delars Derra, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.