Short Communication - (2023) Volume 9, Issue 4
EML4-ALK, an aberrant fusion gene, has been identified in approximately 4%-7% in non-small cell lung cancer. It encodes a cytoplasmic chimeric protein with constitutive kinase activity. Crizotinib, an oral inhibitor of the EML4-ALK kinase activity was de first molecule that showed and important anti tumoral activity in ALK positive non-small cell lung cancer and showed an improvement in overall survival compared to standard chemotherapy [1]. Alectinib, another potent inhibitor of the EML4-ALK kinase, with Central Nervous System (CNS) penetration was also superior to standard chemotherapy as second line and showed superior efficacy compared with crizotinib in the first line setting becoming the standard of care in this scenario. Ceritnib, brigatinib and lorlatinib are also effective molecules for the treatment of patients with ALK positive non-small cell lung cancer. We present the characteristics and treatment outcomes of ALK positive patients treated in the Guatemalan Social Security Institute (IGSS) [2,3].
Of the universe of patients of the medical oncology department of our institution, from January 2013 to December 2020, 269 patients with thoracic tumors were treated [4,5]. 240 were diagnosed with non-small cell lung cancer, identifying 10 patients with EML4-ALK gene fusion and its protein, corresponding to an incidence of 4.16%. Patients characteristics are shown in Table 1.
| Characteristics | No. (%) |
|---|---|
| Age (Years) | |
| Median | 54 |
| Range | (28-62) |
| Gender | |
| Male | 7 (70) |
| Female | 3 (30) |
| Smoking exposure | |
| Never exposed | 8 (80) |
| Exposed | 2 (20) |
| Histologic type | |
| Adenocarcinoma | 8 (80) |
| Bronchioloalveolar | 1 (10) |
| Adenosquamous | 1 (10) |
| Diagnostic method | |
| FISH | 6 (60) |
| IHC | 4 (40) |
| SNC metastases | |
| At diagnosis | 3 (30) |
| At progression | 1 (10) |
| Treatment | |
| Hole brain radiotherapy | 4 (100) |
| Previous chemotherapy | |
| Platinum based duplet | 8 (80) |
| Monotherapy | 1 (10) |
| No chemotherapy | 1 (10) |
| Cycles average | 5 |
| Range | (2-12) |
| Chemotherapy response | |
| No response | 6 (66.7) |
| At least 30% | 3 (33.3) |
| Anti-ALK treatment | |
| 1st line | |
| Crizotinib | 10 (100) |
| 2nd line | |
| Alectinib | 2 (20) |
| Ceritinib | 2 (20) |
| 3rd line | |
| Alectinib | 1 (10) |
Table 1. Characteristics of patients.
All 10 patients received EML4-ALK protein inhibitors at the moment when de alteration was determined (anti-ALK treatment). 6 patients progressed with a progression free survival of 20.0 months (6.80-52.34). 4 patients received a second line with anti-ALK treatment and 1 patient developed a second progression starting anti-ALK treatment as third line [6,7]. At the time of the analysis 3 patients had died. Median overall survival of the group is 32.27 months (9.7-80.28) (Figures 1 and 2).
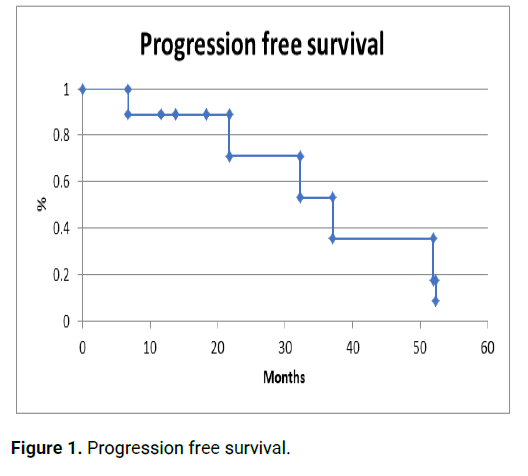
Figure 1: Progression free survival.
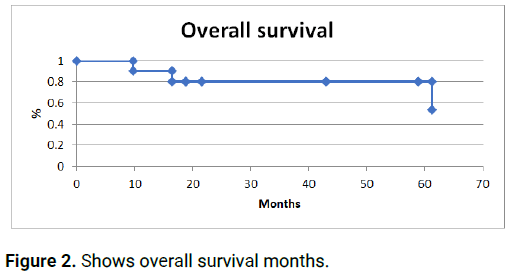
Figure 2: Shows overall survival months.
Lung cancer continues to be the leading cause of cancer deaths worldwide. Since the identification of oncogenic alterations like mutations in the gene encoding Epidermal Growth Factor Receptor (EGFR), translocations in Rat Osteosarcoma (ROS1), expression of Programmed Death Ligand 1 (PD-L1) and EML4-ALK fusion gene, survival has been improved with different therapies targeting these alterations [8,9]. EML4-ALK, an aberrant fusion gene, has been identified in approximately 4%-7% in non-small cell lung cancer. Crizotinib was the first anti-ALK treatment that showed an important activity in patients with ALK positive nonsmall cell lung cancer with response rates of 57%, and had a superior efficacy in previously treated patients compared with chemotherapy and also in previously untreated patients with a median progression free survival of 10.9 months versus 7.0 months compared with chemotherapy [10,11]. Second generation ALK inhibitors like alectinib showed a benefit after crizotinib failure and penetration to CNS with a brain metastases response rate of 33% to 57%. In a randomized trial (ALEX trial) alectinib showed superior efficacy as compared with crizotinib and a lower toxicity rates in the first line treatment of ALK-positive nonsmall cell lung cancer, becoming de standard of care in this scenario. Our ALK positive patients had the characteristics of the patients described in the literature (mostly younger men, non smokers and a high incidence of brain metastases). Although alectinib is the treatment of choice in the first line setting in ALK positive lung cancer all of our patients use crizotinib as a first line treatment. However, they achieved an important progression free survival of 20 months [12,13].
EML4-ALK rearrangement is a rare alteration that define a distinctive clinical and molecular subtype of lung cancer that is more prevalent in younger patients without a smoking exposure, high incidence of CNS metastases and poor prognosis. However, with the development of inhibitors of its kinase activity these kind of patients should receive an anti ALK treatment for an improvement of their clinical outcomes and survival as we have seen in our patients.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Garcia-Aceituno L, et al. "Institutional Experience and Survival Analysis of ALK Positive Non- Small Cell Lung Cancer". Oncol Cancer Case Rep, 2023, 9(4), 1-2.
Received: 07-Apr-2021, Manuscript No. OCCRS-23-111823; Editor assigned: 12-Apr-2021, Pre QC No. OCCRS-23-111823 (PQ); Reviewed: 27-Apr-2021, QC No. OCCRS-23-111823; Revised: 07-Jul-2023, Manuscript No. OCCRS-23-111823 (R); Published: 04-Aug-2023, DOI: 10.4172/2471-8556.23.9.4.003
Copyright: © 2023 Garcia-Aceituno L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.