Research - (2020) Volume 10, Issue 4
Background: Hemogram derived inflammatory markers are being studied in various inflammatory conditions. Liver steatosis is also an inflammatory process, therefore, we aimed to study platelet distribution width (PDW), neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR) and monocyte to lymphocyte ratio (MoLR) in patients with hepatosteatosis and to compare to those in healthy subjects. Methods: Medical data of the patients with hepatosteatosis that diagnosed in outpatient clinics of our institution were obtained and they were enrolled to the study as study group. Heathy volunteers were also enrolled to the study as control group. General characteristics and laboratory data, including PDW, NLR, MoLR and PLR, of the study and control groups were compared. Results: Both PDW (p<0.001), NLR (p=0.01), and MoLR (p=0.02) of the study group was significantly higher than those of the control group. However, PLR of the NAFLD (112, 52%) group was not statistically different from the PLR of the control (110, 39%) subjects (p=0.42). PDW was significantly and positively correlated with fasting blood glucose (r=0.244, p=0.001) and alanine transaminase (ALT) levels (r=0.241, p=0.001). NLR was also significantly and positively correlated with ALT (r=0.18, p=0.01). PDW values higher than 16.25% have 83% sensitivity and 57% specificity in predicting liver steatosis. Conclusion: We suggest that elevated PDW, NLR and MoLR levels should alert physician for liver steatosis in an otherwise healthy subjects and should prompt evaluation of liver tests and imaging studies. Since PDW has high sensitivity and specificity in prediction of liver steatosis, it may be useful in the diagnosis of hepatic steatosis in primary care setting.
Liver steatosis and non-alcoholic fatty liver disease (NAFLD) have prevalence with gradual increase, especially in developed countries. It makes an important proportion of hepatosteatosis and effects almost one third of general population [1]. NAFLD could present as liver steatosis, steatohepatitis or cirrhosis [2]. Insulin resistance, hypertension, metabolic syndrome, obesity and dyslipidemia have close relation with liver steatosis [3].
Numerous indices derived from routine hemogram tests have been suggested as novel markers of inflammation in various inflammatory conditions. Of these, red cell distribution width (RDW) and mean platelet volume (MPV) has been shown to be increased in subjects with irritable bowel syndrome, a condition that associated with low grade inflammation compared to healthy population [4]. Some other hemogram indices, including platelet distribution width (PDW), neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), and monocyte to lymphocyte ratio (MoLR) are also suggested as inflammatory predictors in certain diseases, such as, type 2 diabetes mellitus, cardiac conditions, thyroiditis, chronic obstructive pulmonary disease, familial Mediterranean fever, cancers, osteoporosis, osteoarthritis and rheumatoid arthritis [5-18].
Data in the literature suggests the relationship between hepatosteatosis and inflammatory markers, therefore, we hypothesized that whether PDW, NLR, PLR and MoLR were associated with liver steatosis. For this purpose we compared the hemogram parameters of patients with hepatosteatosis to those in healthy volunteers.
After obtaining approval from institutional board (date: 23rd of August in 2019, number: 33443051/903.99), we retrospectively analyzed the data of patients with NAFLD whom presented to our outpatient clinic between September 2019 and June 2020. Control group was consisted of healthy volunteers that visited our clinic for a routine check-up. Subjects with active infection, inflammatory conditions and cancer were excluded from the study. Subjects with a recent history of trauma or surgery (within a month) were also excluded. Finally, patients on medications that may interfere with platelet or leukocyte function (i.e. steroids, aspirin) were not enrolled to the study. None of the subjects in study and control groups had a history of use of medications. Patients with NAFLD had no history for alcohol consumption and had negative serology for known hepatitis viruses. The presence and stage of liver steatosis was determined by ultrasound scan.
Age, gender, serum uric acid, LDL cholesterol, HDL cholesterol, triglyceride, fasting blood glucose (FBG), creatinine, aspartate and alanine transaminases (AST and ALT) and creactive protein (CRP) levels were recorded from the patients’ files and database of the institution. Hemogram parameters, such as, white blood cell count (WBC), neutrophil count (neu), lymphocyte count (lym), monocyte count (mono), hemoglobin (Hb), hematocrit (Htc), mean red cell volume (MCV), platelet count (plt), platelet distribution width (PDW) were also obtained and recorded. NLR, PLR and MoLR were calculated by the formulas neu/lym, plt/lym and mono/lym, respectively. Laboratory tests of the NAFLD patients at the time of diagnosis were used for analysis. Biochemical analyses were held with Architect c8000 analyzer (Abbott Inc. Lake Forest, IL, USA). Automatic analyzer of LH 780 model of Beckman Coulter device (Beckman Coulter Inc.; Bre CA) was used for complete blood count analyses. Data of NAFLD patients and control subjects were compared.
Data were analyzed with SPSS software (SPSS 15.0; SPSS Inc., Chicago, IL, USA). Distribution of the variables in study and control groups was analyzed with Kolmogorov-Smirnov test. Variables with normal distribution were compared with independent samples t test and expressed as mean ± standard deviation (SD). Mann- Whitney U test is used in comparison of the variables without normal distribution and these variables were expressed as median (IQR). Chi-square test is used in comparison of categorical variables between study and control subjects. Correlation between studies variables were conducted with Pearson’s correlation analysis. Receiver Operative Characteristics (ROC) analyze used in determination of cut-off values of study variables in predicting NAFLD. Statistical significance was set when the p value was lower than 0.05.
The study population consisted of 99 NAFLD patients and 95 control subjects after exclusion criteria were applied. Median ages of the NAFLD and control subjects were 41 (17) and 40 (16) years, respectively (p=0.3). 48 (48.5%) of NAFLD group were women and 51 (51.5%) were men, while 43 (45%) of control group were women and 52 (55%) were men. The gender balance of the study and control groups was not statistically different (p=0.65).
WBC (p=0.31), neu (p=0.73), lym (p=0.41), mono (p=0.18), Hb (p=0.16), Htc (p=0.07), MCV (p=), plt (p=0.47), AST (p=0.87), HDL cholesterol (p=0.73) and triglyceride (p=0.15) of the study and control groups were not statistically different. On the other hand, serum creatinine (p=0.001), ALT (p<0.001), FBG (p<0.001), LDL (p=0.001) and CRP (p<0.001) levels of the study and control groups were statistically different. Data of the NAFLD and control groups were summarized in Table 1.
| NAFLD group | Control group | p | ||
|---|---|---|---|---|
| Gender | Women (n, %) | 48 (48.5%) | 43 (45%) | 0.65 |
| Men (n, %) | 51 (51.5%) | 52 (55%) | ||
| Mean ± SD | ||||
| WBC (k/mm3) | 7.1 ± 1.7 | 7.4 ± 1.7 | 0.31 | |
| Lym (k/mm3) | 2.3 ± 0.9 | 2.4 ± 0.7 | 0.41 | |
| Mono (k/mm3) | 0.6 ± 0.2 | 0.5 ±0.2 | 0.18 | |
| Hb (g/dL) | 14.5 ± 1.5 | 14.8 ±1.4 | 0.16 | |
| Htc (%) | 43 ± 4 | 44 ± 4 | 0.07 | |
| Plt (k/mm3) | 257 ± 90 | 249 ± 56 | 0.47 | |
| PDW (%) | 17.2 ± 1.5 | 15.4 ± 2.2 | <0.001 | |
| NLR (%) | 2.6 ± 0.9 | 1.97 ± 0.8 | 0.01 | |
| Median (IQR) | ||||
| Age (years) | 41 (17) | 40 (16) | 0.3 | |
| PLR (%) | 112 (52) | 110 (39) | 0.42 | |
| MoLR (%) | 0.26 (0.15) | 0.22 (0.11) | 0.02 | |
| Neu (k/mm3) | 4.2 (2.7) | 4.3 (2.2) | 0.73 | |
| Uric acid (mg/dL) | 5 (1.1) | 3 (1) | <0.001 | |
| HDL (mg/dL) | 43 (15) | 42 (10) | 0.73 | |
| MCV (fL) | 86 (4) | 87 (6) | 0.18 | |
| AST (U/L) | 26 (13) | 28 (11) | 0.87 | |
| ALT (U/L) | 41 (27) | 29 (10) | <0.001 | |
| FBG (mg/dL) | 103 (30) | 78 (9) | <0.001 | |
| Creatinine (mg/dL) | 0.8 (0.1) | 0.7 (0.1) | 0.001 | |
| Triglyceride (mg/dL) | 196 (20) | 123 (32) | 0.15 | |
| LDL (mg/dL) | 143 (33) | 129 (33) | 0.001 | |
| CRP (mg/L) | 3.5 (2.7) | 2.6 (1.4) | <0.001 | |
Table 1: Data of NAFLD and control subjects.
The PDW value of the NAFLD and control groups were 17.2 ± 1.5% and 15.4 ± 2.2%, respectively (p<0.001). NLR of the NAFLD and control groups were 2.6 ± 0.9% and 1.97 ± 0.8%, respectively (p=0.01). MoLR of the NAFLD and control groups were 0.26 (0.15)% and 0.22 (0.11)%, respectively (p=0.02). However, PLR of the NAFLD (112, 52%) group was not statistically different from the PLR of control (110, 39%) subjects (p=0.42).
The PDW was significantly and positively correlated with FBG (r=0.244, p=0.001) and ALT (r=0.241, p=0.001) levels. NLR was also significantly and positively correlated with ALT (r=0.18, p=0.01), but not correlated with FBG levels. Neither FBG nor ALT levels were correlated with MoLR values.
In Roc analysis, PDW values higher than 16.25% have 83% sensitivity and 57% specificity in predicting liver steatosis. Moreover, NLR higher than 1.78% has 58% sensitivity and 47% specificity in prediction of hepatic steatosis. Sensitivity and specificity of MoLR higher than 0.22% in predicting liver steatosis were 67% and 49%, respectively. Figure 1 shows the ROC curves of PDW, NLR and MoLR.
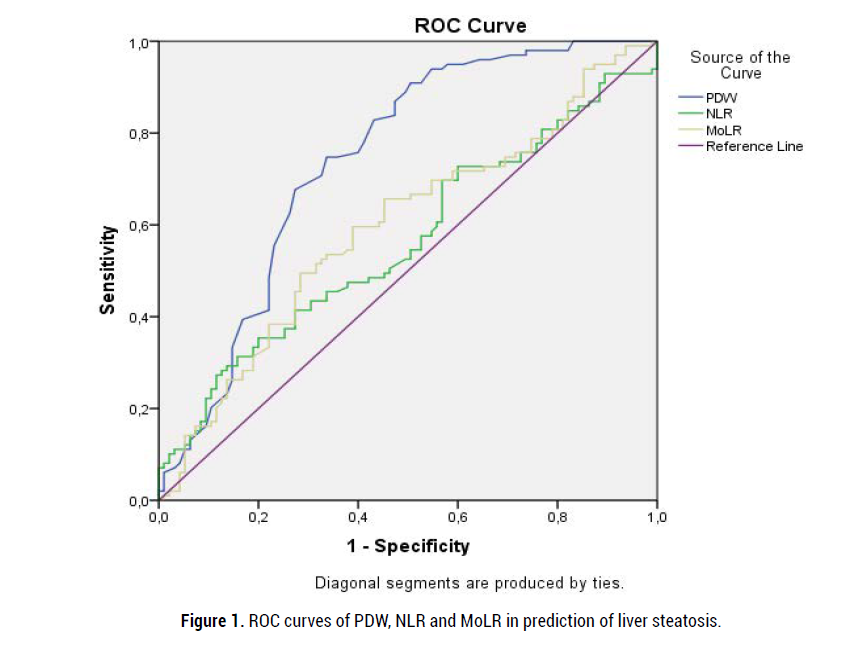
Figure 1: ROC curves of PDW, NLR and MoLR in prediction of liver steatosis.
Interesting outcomes of the present study are (a) significant association between hepatosteatosis and three hemogram derived indices; PDW, NLR and MoLR, (b) moderate correlations between PDW and ALT and between PDW and FBG levels and weak correlation between NLR and ALT levels, and (c) higher sensitivity and specificity for PDW, compared to those in MoLR nd NLR, in predicting liver steatosis.
The NAFLD and its all clinical presentation forms; liver steatosis, steatohepatitis and cirrhosis, are all associated with inflammation [19-21]. Inflammation plays pivotal role in initiation and progression of these hepatic conditions. C-reactive protein (CRP), the most useful marker of inflammatory and infectious conditions, is reported to be elevated in heptic steatosis [22]. Therefore, we may assume that, as well as CRP, other inflammatory markers may also be associated with NAFLD. Hemogram derived inflammatory markers have been studied in various clinical conditions. Of these, erythrocyte distribution width (RDW) and mean platelet volume (MPV) were suggested to be increased in patients with liver steatosis [23]. In another study MPV to platelet ratio has been found to be elevated in subjects with NAFLD [24]. In accordance with literature data, in present study we studied MoLR, NLR and PDW in hepatosteatosis and found that both three parameters were increased compared to those in healthy subjects.
Associations of NLR with other inflammatory conditions have been reported in literature. It was suggested to be related with cancer associated inflammation [25,26]. NLR’s role in metabolic syndrome [27], cardiac conditions [28], in Behçet’s disease [29], in pneumonia [30] and in exacerbation of chronic obstructive pulmonary disease [31] have been well established in medical literature. NLR was not only supposed as a predictor of fibrosis [32], but also claimed as a predictor of liver histology in subjects with NAFLD [33]. Similarly, we found that NLR was increased in patients with hepatic steatosis compared to healthy subjects in present study. Moreover, it was correlated with ALT levels.
Alike with NLR, PDW was also reported to be correlated with specific inflammatory conditions in medical literature. These include obstructive sleep apnea and chronic obstructive pulmonary disease [34], type 2 diabetes mellitus [15], systemic lupus erythematosus [35], stable coronary heart disease [36], non ST elevation myocardial infarction [37], and hypertension [38]. Furthermore, increased levels of PDW were introduced as a prognostic factor in various types of cancer [39-41]. In accordance with literature data, we showed that elevated PDW levels were associated with hepatic steatosis, another condition that related with low but chronic inflammatory burden. Moreover, we showed that it was correlated with ALT and blood sugar levels and has a considerably high sensitivity and specificity in prediction of liver steatosis.
Compared to the NLR and PDW, MoLR is a relatively more novel inflammatory indice that studied in various reports in literature. Gastrointestinal stromal tumor recurrence [42], osteoporosis [18] and gouty arthritis [43] were reported to be related with increased levels of MoLR. In addition, lymphocyte to monocyte ratio, the reverse proportion of MoLR, has been reported to be associated with colorectal cancer [44], coronary heart disease [45], malignant conditions [46,47], and stroke [48]. In present study, we demonstrated that MoLR of the patients with NAFLD was significantly higher than that of the healthy population.
Relatively small study population and design of the study, which is retrospective, are limitations of our work. However, to the best of our knowledge, this is the first study in literature studied NLR, PDW and MoLR together in hepatosteatosis patients.
We suggest that elevated PDW, NLR and MoLR levels should alert physician for liver steatosis in an otherwise healthy subjects and should prompt evaluation of liver tests and imaging studies. Since PDW has high sensitivity and specificity in prediction of liver steatosis, it may be useful in the diagnosis of hepatic steatosis in primary care setting.
Citation: Gulali Aktas, Tuba Taslamacioglu Duman, Özge Kurtkulagi, Burcin Meryem Atak Tel, Satilmis Bilgin, et al. Liver Steatosis is Associated Both with Platelet Distribution Width, Neutrophil/Lymphocyte and Monocyte/Lymphocyte Ratios. Prim Health Care, 2020, 10(4), 001-004.
Received: 26-Jul-2020 Published: 31-Aug-2020
Copyright: 2020 Gulali A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : NA