Research Article - (2024) Volume 13, Issue 1
Objective: Trapeziometacarpal Joint Osteoarthritis (TMJOA) is the second most common degenerative disease of the hand after interphalangeal joint osteoarthtritis. This condition is influenced by aging and comorbidities determining an increase in pharmacological treatment and a high social and economic burden. Natural substances such as Hyaluronic Acid (HA), Chondroitin Sulfate (CS), and glucosamine are starting to be used as ground therapy to reduce the consumption of NSAIDs and their adverse events. Ialòral® 1500 is a natural compound made of biocell collagen, cynantine, manganese, and bioperine. Our objective was to evaluate the effect of a 1-month therapy with Ialòral® 1500 through DASH score and to consider if this treatment leads to pain reduction (NRS score) and strength improvement (Jamar test and Pinch test).
Design: In 3 months, we recruited through X-Ray (XR), Jamar test; Pinch test, NRS, and DASH score 40 patients with TMJOA. Patients assumed once a day for 28 days an oral supplement of 120 mg HA, 240 mg CS, 300 mg K, keratin matrix, manganese, and piperine. NRS and DASH score were assessed at 2, 4, 8 weeks from the beginning (T1, T2, T3) whereas Jamar and Pinch tests were done during T2 and T3 visits.
Results: Our study showed improvement in DASH, NRS scores and in the grip and pincher movements. Results were more significant for the advanced stages.
Conclusion: This oral supplementation may provide an additional non-invasive possibility of treatment of the of TMJOA symptoms, especially for those patients with contraindication to more invasive approaches.
Osteoarthritis thumb • Trapeziometacarpal joint • Hyaluronic acid • Chondroitin sulfate • Piperine • Manganese
TMJ primary osteoarthritis is the second most common degenerative disease of the hand after IPJ osteoarthritis. Patients report pain, stiffness, and impaired hand function in strength and dexterity, refractory to splinting and anti-inflammatory drugs [1,2]. This condition which currently affects 10% of the population, is significantly influenced by aging and comorbidities affecting the population, determining an increase in pharmacological treatment and a high social and economic burden [3]. Genetic and environmental risks such as ligamentous laxity, hormonal alterations, stress, obesity and repeated microtraumatisms might be responsible for a pro-inflammatory status that stimulates proteolytic enzymes, cytokines, reactive oxygen species, and metalloproteinases involved in synovial inflammation and cartilage degeneration. The latter, along with osteophyte formation, subchondral bone sclerosis and synovial, ligamentous, and joint capsule damage are the pathological hallmarks of the disease [4]. The Eaton-Littler is the most frequent used classification and describes four stages of TMJ OA referring to the radiological view. This classification, while having a poor correlation between radiographic findings and symptomatology, helps to recognize painful laxity and differentiate between patients with isolated TMJ arthritis from those in which closer joints are involved. Correct treatment mainly depends on functional demands, occupation, and activities; all treatments aim to reduce pain while increasing mobility and thumb pinch strength [5,6]. While for earlier stages, conservative management with rest, splinting, physiotherapy, oral or topical NSAIDs, and intra-articular steroid or HA injections may be indicated, in most advanced radiological stages, a surgical intervention might be indicated [7]. Interest has been growing in natural substances such as Hyaluronic Acid (HA), Chondroitin Sulfate (CS), and glucosamine that are starting to be used as ground therapy to reduce consumption of NSAIDs and their related adverse events. Ialòral® 1500 (PharmaSuisse Laboratories Srl, Milan, Italy) is a natural compound made of BioCell Collagen, Cynantine, Manganese, and Bioperine like that of the human articular cartilage in synovial joints. Our primary objective was to evaluate its effect after 28 days of therapy (T2) through objective and subjective evaluation of hand function (DASH score), pain (NRS score) and strength (Jamar test and Pinch test). We additionally followed up with our patients to see whether the results were changed throughout the following month (T3).
For 3 months, we selected 40 consecutive patients with TMJOA visiting our Unit. Patients were equally divided into two groups according to the radiological Eaton-Littler classification: Group A stage I-II and group B stage III-IV. Patients with a previous history of TMJ trauma, who recently underwent corticosteroid therapy (oral or infiltrations), in pregnancy, with rheumatological or dysmetabolic diseases, or younger than 18 years were excluded. In patients with both hands affected, the most symptomatic limb was the one considered in our study. The study population consisted of 7 patients in stage I (17,1 %), 14 patients in stage II (34,1 %), 13 patients in stage III (31,7 %), and 7 patients in stage IV (17,1%) according to Eaton-Littler classification. 36.6% (15 patients) dominant hand; 63,4% (26 patients) non dominant hand. Group A consisted of 21 patients and group B of 20 patients. 6 patients were male (14,6%) and 35 were female (85,4%). One patient (female, group A, stage II sec. E-L, dominant handed) dropped out due to other medical issues and was therefore not included in the statistical analysis.
Periodic outpatient evaluations were performed according to the study protocol by two operators, over a 2-month period during which they were recruited at ambulatorial visits. X-rays, NRS score, DASH score, Jamar and Pinch strength tests were run and reported in a digital database [8-10]. During the visit, patients received an oral supplement of 120 mg HA, 240 mg CS, 300 mg K, keratin matrix, manganese, and piperine (Ialòral® 1500, PharmaSuisse Laboratories Srl, Milan, Italy) to take once a day for 28 days. Patients were tested after 2 weeks through NRS and DASH score online evaluations and two more visits were done at 30 and 60 days from enrollment during which Jamar and Pinch tests were noted along with NRS and DASH score evaluations. The study was approved by the Hospital Internal Review Board and carried out according to the principles of the Ethical Declaration of Helsinki. All patients signed an informed consent form to participate in the study. All demographic and clinical data of the analyzed cohort were summarized using descriptive statistical methods. In particular, the mean values and standard deviations were used for the symmetrically distributed continuous variables. The frequencies of treatment-related adverse events were recorded during the study period. Pre/post-treatment differences in grip strength were assessed separately in the two study groups and differences between pain scores and ability to carry out activities of daily living measured at visits T0, T1, T2, T3 and with the online test were assessed separately in the two study groups. All statistical tests were performed considering a significance level of 5%.
No adverse events were detected during the administration of the compound. One dropout was reported due to the patient’s other medical issues, so the patient was therefore not included in statistical analysis. All results for DASH, NRS, Jamar, and KPinch evaluations are summarized in Table 1. The test for the DASH score shows improvement in both groups throughout the visits and in the paired data test (t-test) at T0 and T3. While for group A this improvement was non-statistically significant (p>0.05), in group B the improvement was statistically significant (p<0.05). The average DASH score between to and T2 decreased by a total of 9.2 points.
(from 80.3 ± 9.3 to 71.1 ± 7.5) for group A, and this improvement remained constant over time, including at T3 (71.1 ± 6.8). For group B, there was a statistically significant improvement of 8.3 points between T0 and T2 (from 87.9 ± 7.3 to 79.6 ± 7.5) with a slight regression in improvement at T3 (from 79.6 ± 7.5 to 84 ± 9.2), still retaining an improvement of 3.9 points between T0 and T3. This shows that patients of all stages report an improvement in everyday function that is maintained even after the end of treatment. Figure 1. The test for the NRS score shows a statistically significant improvement in both groups (p<0.05), in the time series, and in the paired data test (Kolmogorov-Smirnov test). The average NRS score in group A between T0 and T2 improved of 1,9 points decreasing from 6 ± 0.8 points to 4.1 ± 1.3 points, with further symptom improvement at T3, with an average value of 3.6 ± 0.8. The same trend occurred in group B, where we went from 7.1 ± 0.8 at T0 to 5 ± 0.8 at T2, with additional improvement at T3 to 4.2 ± 0.8 for an overall improvement of 2.9 points. This value was statistically significant for both groups, meaning that pain reduction after treatment was obtained and maintained during the following month (Figure 2). JAMAR score in group A showed a nonstatistically significant improvement (p>0.05) in the time series. In group B on the other side, the improvement was statistically significant (p<0.05). The average Jamar score between T0 and T3 increased from 16.6 ± 2. 9 to 18.9 ± 2.5 for group A and from 17.4 ± 4.2 at T0 to 19.9 ± 4.7 at T3 for group B. While for group B this finding was statistically significant, the lack of significance in the Friedman test in group A could be attributed to the limited sample size (Figure 3). Finally, the PINCH score shows a statistically non-significant improvement (p>0.05) in group A whereas in group B the improvement is statistically significant (p<0.05). The average Pinch score between T0 and T3 increased from 3.7 ± 0.6 to 3.8 ± 0.6 for group A which was non-significant while group B improved significantly from 2.8 ± 0.8 at T0 to 3.9 ± 1 at T3. It's important to note that the average improvement for group A is smaller than the estimated instrument’s precision (0.5) which means that improvement in thumb grip cannot be proven without doubt in group A (Figure 4).
| DASH score | T0 | T1 | T2 | T3 |
|---|---|---|---|---|
| Group A | 80.25 ± 9.3157 | 71.8 ± 8.0199 | 71.050 ± 7.5335 | 71.05 ± 6.7626 |
| Group B | 87.85 ± 7.2874 | 78.2 ± 8.3135 | 79.600 ± 7.5211 | 83.95 ± 9.2081 |
| Tot | 84.05 ± 5.7739 | 75.0 ± 5.6062 | 75.325 ± 5.2628 | 77.50 ± 5.8359 |
| NRS score | T0 | T1 | T2 | T3 |
| Group A | 5.950 ± 0.8382 | 5.45 ± 0.9171 | 4.05 ± 1.3279 | 3.55 ± 0.8244 |
| Group B | 7.100 ± 0.7577 | 5.75 ± 0.6597 | 4.95 ± 0.7959 | 4.15 ± 0.8074 |
| Tot | 6.525 ± 0.5702 | 5.60 ± 0.5410 | 4.50 ± 0.7527 | 3.85 ± 0.5589 |
| JAMAR score | T0 | T1 | T2 | T3 |
| Group A | 16.55 ± 2.8789 | - | 18.900 ± 2.0423 | 18.85 ± 2.4591 |
| Group B | 17.35 ± 4.1778 | - | 18.950 ± 4.8625 | 19.85 ± 4.6611 |
| Tot | 16.95 ± 2.4234 | - | 18.925 ± 2.5155 | 19.35 ± 2.5188 |
| PINCH score | T0 | T1 | T2 | T3 |
| Group A | 3.6535 ± 0.5653 | - | 3.3282 ± 0.5344 | 3.7602 ± 0.6026 |
| Group B | 2.7592 ± 0.7951 | - | 3.2835 ± 0.8716 | 3.8445 ± 0.9503 |
| Tot | 3.2063 ± 0.4873 | - | 3.3058 ± 0.4877 | 3.8023 ± 0.5369 |
Table 1. All results for DASH, NRS, Jamar, and K-Pinch evaluations are summarized.
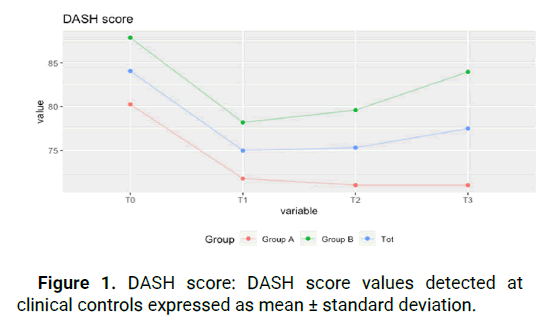
Figure 1: DASH score: DASH score values detected at clinical controls expressed as mean ± standard deviation.
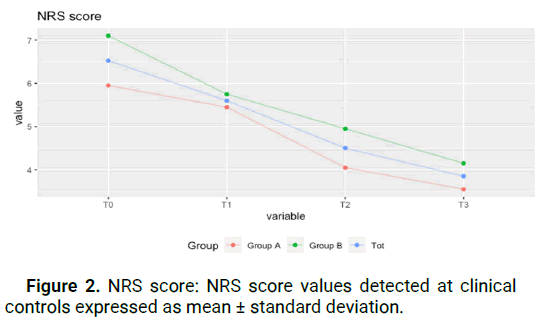
Figure 2: NRS score: NRS score values detected at clinical controls expressed as mean ± standard deviation.
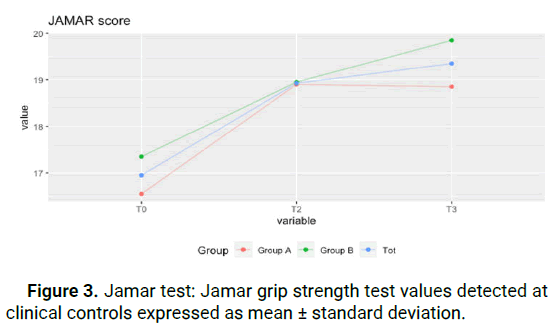
Figure 3: Jamar test: Jamar grip strength test values detected at clinical controls expressed as mean ± standard deviation.
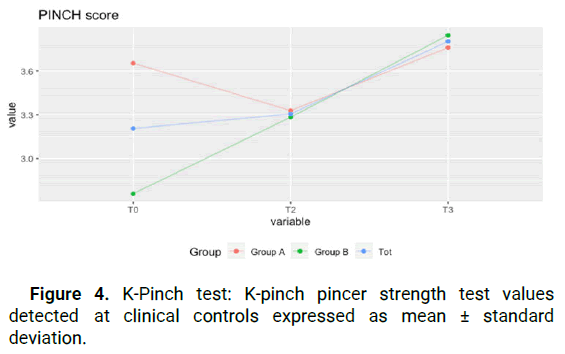
Figure 4: K-Pinch test: K-pinch pincer strength test values detected at clinical controls expressed as mean ± standard deviation.
Our study allowed us to evaluate functional and pain improvement after treatment with Ialòral® 1500 in patients affected by TMJOA in different stages. The analyzed cohort was not homogenous and non-normally distributed; no correlations were found between the improvement in the indices and factors such as age and degree of arthritis.
To avoid a further bias, during the design phase of the study protocol, in patients with both hands affected we decided to consider the most symptomatic hand as the one analyzed in our study. Ialòral® 1500 is already described in the treatment of knee chondropaties and osteoarthritis, both preoperatively and postoperatively to maintain the results achieved with infiltrative therapy. Previous studies showed its efficacy after 28 days of treatment [3,11,12]. This compound has not yet been studied in the treatment of TMJ osteoarthrosis. There are limited pharmacological choices for Osteoarthritis (OA), primarily focused on alleviating symptoms like pain and discomfort. Although oral Glucosamine Sulfate (GS) and Chondroitin Sulfate (CS) are widely recommended for OA management, there is scanty evidence regarding the efficacy of oral Hyaluronic Acid (HA) in OA patients. Few randomized, placebo-controlled trials have demonstrated a potential improvement in knee pain and WOMAC scores in these individuals [4]. A limitation of this study is the absence of a control group and the subjective assessment with the DASH scale only, as well as the small number of patients recruited. We found that, the use of Ialòral® 1500 taken orally for 4 weeks has the potential to improve pain symptoms, functional deficits and improve the use of the affected hand in daily activities for patients with TMJ OA. We consider that Ialòral® 1500 represents a valid option to reduce pain and increase the hand’s function in older patients with comorbidities too. Further studies should be conducted to compare outcomes achieved with Ialòral® to results after placebo treatment. Moreover, the size of the cohort should be increased to confirm and reinforce what was detected by our trial. It is not yet defined how long the treatment performed can maintain benefits for the patient, as the long-term follow-up is still ongoing.
In conclusion, TMJ primary osteoarthritis is a significant challenge affecting a considerable portion of the population and imposing a substantial social and economic burden. Traditional treatment options, including splinting and anti-inflammatory drugs, often fall short in providing long-term relief. Our study focused on evaluating the efficacy of Ialòral® 1500, a natural compound, in improving hand function and reducing pain in patients with TMJOA across different stages.
Our findings indicate that treatment with Ialòral® 1500 led to significant improvements in pain levels, hand function, and strength, particularly in patients classified under Eaton-Littler stages III and IV. These improvements were sustained even after the completion of the 28-day therapy period, suggesting a lasting benefit. This oral supplementation may provide an additional non-invasive possibility of treatment of the of TMJOA symptoms, especially for those patients with contraindication to more invasive approaches.
Dr. Massimo Corain provided the concept and helped in the study design as well as in the supervision and critical review of the article. Dr. Maria Carolina Fra was involved in the study design, literature review, and actual writing of the article. Dr. Tommaso Maluta, Dr. Mattia Cason and Dr. Elmor Moore participated in the collection and processing of data and the critical review of the article, whereas Luca Cristani interpreted and analyzed the data extracted.
PharmaSuisse Laboratories SRL. provided the oral compound for all patients selected.
The study was financed by PharmaSuisse Laboratories SRL.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Fra MC, et al. "Outcome of the Treatment of Symptomatic Osteoarthritis of the Trapeziometacarpal Joint with an Oral Compound Containing Hyaluronic Acid, Chondroitin Sulfate, Hydrolyzed Collagen Type II, and Hydrolyzed Keratin in 40 Patients". J Arthritis, 2024, 13(1), 1-4.
Received: 05-Jan-2024, Manuscript No. JAHS-24-124518; Editor assigned: 08-Jan-2024, Pre QC No. JAHS-24-124518 (PQ); Reviewed: 22-Jan-2024, QC No. JAHS-24-124518; Revised: 05-Mar-2024, Manuscript No. JAHS-24-124518 (R); Published: 12-Mar-2024, DOI: 10.35248/2167-7921.24.13(1).104
Copyright: © 2024 Fra MC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.