Case Report - (2023) Volume 8, Issue 1
Achalasia is a rare esophageal motility disorder defined by absent peristalsis and failure of the lower esophageal sphincter (LES) to relax. Lack of LES relaxation results in symptoms of dysphagia, weight loss, and regurgitation. Exact pathophysiology of the degeneration leading to achalasia is unclear, but autoimmune, viral, or inflammatory neurodegenerative processes are suspected. In this case, we present a 37-year-old male with a history of pneumonia related to pulmonary actinomycosis followed by symptoms of achalasia. The patient was managed by antibiotics and surgical intervention.
Actinomycosis • Achalasia • Pneumonia • Dysphagia • Neurodegenerative
Achalasia has been regarded as an uncommon disorder with an estimated annual prevalence rate of 10 cases per 100,000 individuals [1]. Dysphagia, regurgitation of undigested food, chest pain, cachexia, and respiratory complications are common clinical manifestations of achalasia due to aperistalsis and impaired swallow-induced relaxation of the LES. Evidence has emerged showing the functional abnormalities are a result of ganglion cells degeneration in the myenteric plexus of the esophageal body, however the triggering pathogenesis behind the loss of motor innervation is still elusive [2]. Several reports have suggested an underlying etiological factor is immune-mediated inflammation associated with infectious disease, leading to progressive destruction of the inhibitory myenteric neurons [3]. Histological and immunochemical studies of viral and bacterial induced inflammation in patients with achalasia showed inflammatory infiltrates CD3+, CD4+, CD25+, and CD8+ T lymphocytes around myenteric neurons compared to control groups with no visible infiltrates and normal myenteric plexus [4]. A proposed model of achalasia pathophysiology suggests these inflammatory infiltrates of chronic bacterial or viral infection have a predilection for squamous cell epithelium and neurons limited to the esophagus, which results in the clinical features of achalasia [5]. Under the same model, aggressive inflammatory response involving Th1, Th2, T and B regulatory immune cells results in wound repair fibrosis that progresses to a loss of immunological tolerance in individuals with genetic predisposition [6]. With uncertainty around the exact pathophysiology behind the cause of achalasia, therapeutic treatments focus on ameliorating symptoms rather than preventative or cure of the disease. Treatment options available today include pharmacotherapy, botulinum toxin injection, endoscopical dilatation, esophageal stent, and surgical myotomy to improve esophageal outflow obstruction [7,8].
A 37-year-old Caucasian male with no significant past medical condition, was previously a smoker but discontinued in November 2020 presented with a history of pneumonia concurrent with hemoptysis for a year and a half. Patient was initially treated as an outpatient in the emergency room for pneumonia with oral erythromycin but was subsequently admitted to the intensive care unit after treatment failure and worsened condition. During hospitalization, the patient became progressively worse and was submitted for a bronchoscopy with biopsy. Microbiologic examination isolated Actinomyces israelii and the patient was diagnosed with actinomycosis. Following the patient’s recovery from pulmonary actinomycosis, he developed progressive dysphagia, and a modified barium swallow was performed, which showed a bird beak sign, indicating a tapered distal esophagus that is characteristic of achalasia (Figure 1). An Esophagogastroduodenoscopy (EGD) was consequently performed and showed classic-appearing achalasia. Symptoms subsided after balloon dilatation but recurred three to four weeks later and he presented in office with mid-chest discomfort. Patient reports a weight loss from 185 pounds to 172 pounds with nocturnal emesis four to five times per night. An esophageal motility study was performed, and the patient elected to undergo a laparoscopic Heller cardiomyotomy.
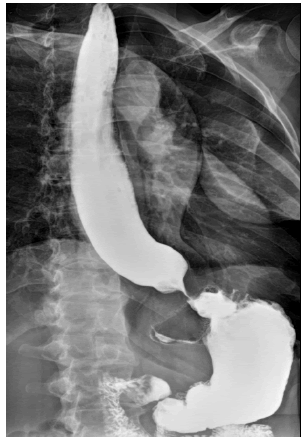
Figure 1: Barium swallow study displaying bird beak sign [5]
Inhibitory neurons located in the distal esophageal wall and LES release Vasoactive Intestinal Polypeptide (VIP) and Nitric Oxide (NO) resulting in esophageal relaxation, while excitatory neurons release acetylcholine for contraction [9]. Achalasia is a rare esophageal disease characterized by motor dyskinesia that arise because of myenteric inhibitory neuron destruction within the LES [9]. Although well-defined and widely studied, the exact etiology behind the gradual disappearance of inhibitory neurons remains ambiguous [10]. Previous studies have revealed inflammatory cell infiltration in the Auerbach’s plexus as a common denominator for the pathogenesis of achalasia, which falls under three categories: autoimmune, genetic, and infection [11]. In our case study, the patient presented with pulmonary actinomycosis, often caused by Actinomyces israelii, an anaerobic, gram-positive prokaryotic bacteria found in the human oral, digestive, and genital tracts [12]. Pulmonary involvement usually stems from aspiration of oropharyngeal or gastrointestinal secretions into the respiratory tract [12]. Primary interest of this case is due to limited reports on esophageal disorders associated with pulmonary actinomycosis and the unusual occurrence of achalasia post-infection [13]. In other documented sources, pulmonary actinomycosis results from aspiration of contaminated secretions into the bronchial tree and a common source is regurgitated gastric content from patients diagnosed with achalasia [14]. However, in our patient, symptoms of nocturnal emesis associated with achalasia developed following his recovery from pulmonary actinomycosis.
Our understanding regarding the etiology of achalasia continues to grow and each theory points to an inflammatory process that triggers the degeneration of inhibitory myenteric neurons in the LES. While some cases have documented an association of cardia achalasia leading to aspiration pneumonia, our case encountered a reversed presentation of pulmonary actinomycosis resulting in achalasia. This adds to the theory that loss of VIP and NO secreting neurons could be a result of an inflammatory response. Ultimately, more research is needed regarding the exact pathogenesis of achalasia and whether it is associated with infection for effective therapy.
[Google scholar] [Cross ref] Circ: Cardiovasc Imagin
Citation: Asbury, C., et al. Pulmonary Actinomycosis and Achalasia: Cause or Consequence? Case Report. Med Rep Case Stud. 2023, 08 (1),001-002
Received: 21-Jan-2023, Manuscript No. MRCS-22-87582; Editor assigned: 23-Jan-2023, Pre QC No. MRCS- 22- 87582 (PQ); Reviewed: 01-Feb-2023, QC No. MRCS- 22- 87582 (Q); Revised: 04-Feb-2023, Manuscript No. MRCS- 22- 87582 (R); Published: 06-Feb-2023, DOI: 10.4172/2572 5130.23.8(1).1000235
Copyright: ©2023 Asbury, C et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.