Case Report - (2024) Volume 10, Issue 3
Relapsed/refractory (r/r) Mantle Cell Lymphoma (MCL) is a subtype of mature B-cell lymphoma and characterized by heterogenous and aggressive clinical behaviors. Patients treated with conventional agents have a poor prognosis, especially those who have failed BTKi treatment, indicating a high unmet need for novel therapies. Chimeric Antigen Receptor (CAR) T-cell therapy has been approved for the treatment of r/r hematologic malignancies. In this case, we present a patient with MCL who exhibited many poor risk features, such as a high tumor burden with a Sum of Product of Diameters (SPD) of 19124 mm2, serous cavity effusion, and extensive treatment with systematic therapies. After failing multiple lines of systemic chemotherapies, autologous stem-cell transplantation, and treatment with the BTK inhibitor (ibrutinib), the patient received a single dose of 100 × 106 CAR+ T cells of relma-cel, a cellular therapy comprising autologous T cells transduced with an anti-CD19 chimeric antigen receptor. The patient responded to the relma-cel treatment with a long-term and sustainable Complete Response (CR), which persisted until the end of the 2-year follow-up period. Our findings confirmed the therapeutic benefit of relma-cel in r/r MCL patients with high-risk features.
Chimeric antigen receptor T-cell (CAR-T) therapy • Mantle Cell Lymphoma (MCL) • Case report • Relmacabtagene autoleucel (Relma-cel)
Mantle Cell Lymphoma (MCL) is a rare subtype of B-cell NHL with high heterogeneity and an extremely poor prognosis. Although significant clinical efficacy in the treatment of patients with r/r MCL has been shown with Bruton Tyrosine Kinase inhibitors (BTKis) [1], the disease is not curative with the currently available therapies, and most patients eventually experience disease progression. Therefore, there is an unmet need for novel strategies to improve the outcomes of r/r MCL patients.
Chimeric Antigen Receptor T-cell (CAR-T) therapy (Brexucabtagene autoleucel, Brexu-cel) has been approved for r/r MCL by Food and Drug Administration (FDA) in the United States [2]. In China, several products, including relma-cel, are currently being investigated in clinical studies, and not commercially approved yet for the treatment of r/r MCL. Relma-cel has been commercially approved in China in the treatment of patients with r/r LBCL or r/r FL who have failed two or more prior lines of therapies.
A single-arm, open-label, multi-center, phase II study (NCT04718883) is currently underway in China to evaluate the efficacy and safety of relma-cel in r/r MCL. In this report, we present the case of a patient with r/r MCL who had multiple high-risk factors, achieved durable complete remission for more than 48 months, and exhibited good tolerance to relma-cel therapy.
A 57-year-old male patient with a history of nasosinusitis for more than 10 years was initially diagnosed with Ann-Arbor Stage IIIS MCL in January 2013 and was treated with CAR-T approximately 8 years later (Table 1). The initial abdominal CT scan showed that the lymphoma involved both kidneys and bilateral inguinal lymph nodes with splenomegaly. Immunohistochemical analysis of tumor tissue revealed the presence of CD20 (+), CycIin-D1 (+) and Ki-67 (10%) while molecules such as CD3ε, CD5, CD10, CD23, CD43 and BCL2 were negative. He achieved a Complete Response (CR) after firstline treatment with 6 cycles of R-CHOP chemotherapy, and subsequently underwent pretreatment with high-dose therapy (R-CEAC) for Autologous Stem-Cell Transplantation (ASCT) as consolidation therapy. This patient remained in remission until developing Progressive Disease (PD) in August 2015. The PD persisted in the patient despite 6 cycles of bortezomib in combination with cyclophosphamide, etoposide, and DE, 2 cycles of GDP, 2 cycles of lenalidomide, BTKi (Ibrutinib), 5 cycles of bendamustine, and BTKi (zanubrutinib) (Figure 1). Overall, the patient received 7 lines of prior therapies before CAR-T infusion.
| Baseline/outcomes | Data (Data cutoff of 25-Oct, 2023) |
|---|---|
| Age/Sex | 57/M |
| Ki67 | 25% |
| Disease stage | IVa |
| Prior lines of therapy | 7 |
| ECOG | 0 |
| MIPI | 4 |
| SPD (mm2) | 19124 |
| Bulky disease (cm) | 11.2 |
| Bone marrow involvement | Yes |
| Extranodal Lesion | Yes |
| Time from diagnosis to CART therapy (months) | 101 |
| Time between last therapy and relma-cel therapy (days) | 51 |
| CAR+ T cell dose (× 106) | 100 |
| CRS (Grade) | 2 |
| NT (Grade) | Not experienced |
| Grade 3/4 AEs | Pneumonia, Bacteraemia, Aspartate aminotransferase increased, Neutrophil count decreased, Hypocalcaemia, White blood cell count decreased, Lymphocyte count and no grade 4 AEdecreased. |
| Response at 3 Months | CR |
| PFS (months) | >2 Years |
| OS (months) | >2 Years |
| Note: AE: Adverse Event; CR, Complete Response; CRS: Cytokine Release Syndrome; ECOG: Eastern Cooperative Oncology Group; M: Male; MIPI: MCL International Prognostic Index; NT: Neurotoxicity; OS: Overall Survival; PFS: Progression-Free Survival; SPD: Sum of Perpendicular Diameters | |
Table 1. Baseline characteristics and outcomes.
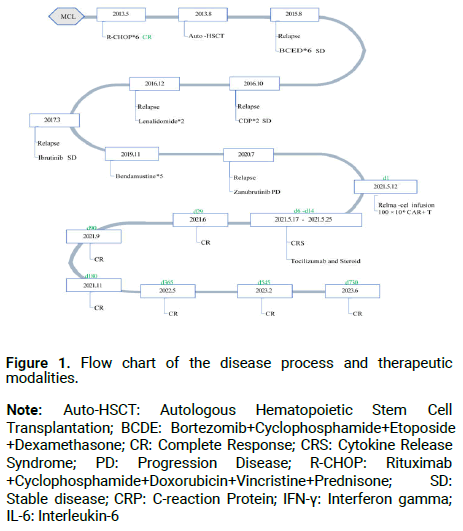
Figure 1: Flow chart of the disease process and therapeutic modalities.
Note: Auto-HSCT: Autologous Hematopoietic Stem Cell Transplantation; BCDE: Bortezomib+Cyclophosphamide+Etoposide +Dexamethasone; CR: Complete Response; CRS: Cytokine Release Syndrome; PD: Progression Disease; R-CHOP: Rituximab +Cyclophosphamide+Doxorubicin+Vincristine+Prednisone; SD: Stable disease; CRP: C-reaction Protein; IFN-γ: Interferon gamma; IL-6: Interleukin-6
In March 2021, this patient was enrolled into the study of relma-cel for r/r MCL patients conducted at Peking University Cancer Hospital. During the screening period, a Computed Tomography (CT) showed the involvement of bilateral cervical, thoracic, abdominal, iliac vessel and inguinal lymphadenopathy, right pleura, liver and bilateral kidneys, with the longest diameter of 112 mm. Also, the infiltration of the pleura, liver and both kidneys, along with ascites and pleural effusion, was shown. FDG-PET/CT at baseline found right pleural area, splenomegaly, bilateral renal, peritoneal nodules, as well as a sternum and right chest wall intermuscular nodule with a high metabolic lesion. In order to relieve the serous cavity effusion, ultrasound-guided pleural puncture was performed, and pleural effusion was drained with an indentation chest drainage tube. The biochemical examination of serous cavity effusion suggested the presence of mantle cell lymphoma cells. The T-SPOT test result was positive with no obvious symptoms of tuberculosis infection. The bone marrow biopsy indicated infiltration by MCL cells with positive expression of CD20 and CD5. Immunohistochemical analysis of pleural effusion showed SOX-11 (-) and CYCLIND1 (partially weak +). The FISH showed positive results for CCND1 and IGH gene fusion, indicating relapse with MCL that was detected at initial diagnosis. The patient presented with several high-risk features, including bulky disease (the longest diameter of a target lesion was 112 mm), ascites and pleural effusion, a Sum of Perpendicular Diameters (SPD) of 19124 mm2, LDH>1.5 times the upper limit of normal value (ULN), and Ki67 (25%+) (Table 1).
The patient received no bridging therapy during relma-cel manufacture. Before relma-cel infusion at the dose of 100 × 106 CAR+ T cells at day 1, he received a lymphodepleting chemotherapy with fludarabine (25 mg/m2) and cyclophosphamide (250 mg/m2). Pre-leukapheresis symptoms, including bronchopneumonia, cough, yellow and thick sputum, and runny nose lasted until the CT scan showed recovery on the second day of lymphodepletion. The patient began to experience intermittent fever up to 39.5°C as early as the day 6 after infusion and was assessed grade 2 CRS, and was accordingly treated with two doses of tolizumab (548.8 mg, once daily (QD)) and three doses of dexamethasone sodium phosphate injection (10 mg, once daily (QD) for continuous 3 days). The CRS was completely resolved after 8 days, and no NT of any grade was diagnosed per ASTCT 2019 [3].
The pharmacokinetic profile of relma-cel in Peripheral Blood (PB) was determined based on the transgene copy numbers per microgram of DNA using qPCR and CD3+ CAR+ cell numbers by flow cytometry. In addition, CD19+ B-cells were also detected by flow cytometry. CAR-T cells started to expand at day 4, peaked around day 11 before decreasing, and disappeared after 60 days of infusion (Figure 2A). Persistent B-cell depletion and hypogammaglobinemia had been previously reported in lymphoma patients treated with CD19-targeting CAR-Ts [4]. In this case report, CD19+ B-cells were not initially detectable at day 11 and the depletion persisted up to 60 days after infusion (Figure 2B). After relma-cel therapy, serum IgA and IgM levels were significantly decreased with no recovery up to 2 years (Figure 3A). Serum levels of Interferon Gamma (IFN-γ), Interleukin-6 (IL-6), ferritin and CRP increased after 1-2 weeks of relma-cel infusion (Figures 3B and 3C). The increases in these inflammatory markers and cytokines were associated with relma-cel-mediated cytokine release syndrome.
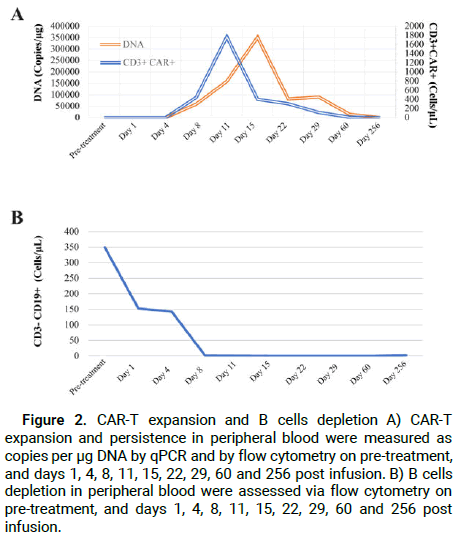
Figure 2: CAR-T expansion and B cells depletion A) CAR-T expansion and persistence in peripheral blood were measured as copies per μg DNA by qPCR and by flow cytometry on pre-treatment, and days 1, 4, 8, 11, 15, 22, 29, 60 and 256 post infusion. B) B cells depletion in peripheral blood were assessed via flow cytometry on pre-treatment, and days 1, 4, 8, 11, 15, 22, 29, 60 and 256 post infusion.
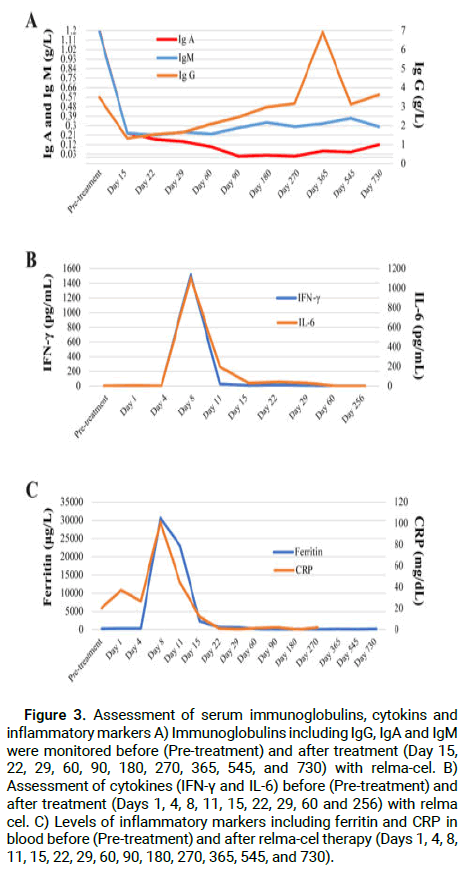
Figure 3: Assessment of serum immunoglobulins, cytokins and inflammatory markers A) Immunoglobulins including IgG, IgA and IgM were monitored before (Pre-treatment) and after treatment (Day 15, 22, 29, 60, 90, 180, 270, 365, 545, and 730) with relma-cel. B) Assessment of cytokines (IFN-γ and IL-6) before (Pre-treatment) and after treatment (Days 1, 4, 8, 11, 15, 22, 29, 60 and 256) with relma cel. C) Levels of inflammatory markers including ferritin and CRP in blood before (Pre-treatment) and after relma-cel therapy (Days 1, 4, 8, 11, 15, 22, 29, 60, 90, 180, 270, 365, 545, and 730).
One month after relma-cel infusion, the overall response indicated sustained complete metabolic remission of the disease. The FDG-PET/ CT performed at day 29 showed no significant disease (SUV max 2.7) compared to the baseline SUV max of 6.2, and reduced ascites and pleural effusion were observed. The CT performed at day 29 showed that multiple enlarged lymph nodes in the neck, chest, abdomen, iliac and inguinal regions were reduced from baseline (the longest diameter of 112 mm to 81 mm). Complete remission lasted more than 2 years as assessed by CT scan after therapy (Figure 4). The residual disease was not detected in the assessment using the bone marrow smear at day 15 after infusion.

Figure 4: Remission status on relma-cel infusion A) FDG-PET/CT scan of target mass prior (above) and after treatment (D29, below). B) Full body FDG-PET scan prior (left) and after treatment (D29, right).
Here, we present a patient with stage IVa MCL with multiple high-risk factors after failing 7 lines of prior therapies. An extremely poor prognosis was therefore obtained for the patient prior to the CAR-T therapy. Desperate for a solution, the patient received CAR-T cell therapy (relma-cel) at the dose level of 100 × 106 CAR+ T cells. The relma-cel reversed the dangerous condition and achieved a sustainable CR for more than 2 years with only grade 2 CRS.
Treated with ibrutinib, the patients with progression showed poor outcome with a median OS of 6 to 8 months [5,6], which was mainly attributed to the more heavily pre-treated nature of the patient. The BTKi-resistant patients often had highly aggressive disease, and 57% patients were unsuitable for any further therapies [7]. In a retrospective cohort study, patients who progressed on BTKi received R-BAC (rituximab, bendamustine, cytarabine) and achieved a median progression-free survival of 10.1 month and a median overall survival of 12.5 months [8].
With the development of anti-CD19 CAR-T therapy, the limited conventional treatment options in the patients with r/r lymphoid malignancies have been revolutionized. According to the publicly disclosed clinical results, CD19 CAR-T therapies exhibited a high response rate (ORR at about 80%) and survival (PFS at about 15 months) in MCL survivors who experienced PD after BTKi treatment [9,10]. While there was no association between the pre-KTE-X19 MCL tumor volume and clinical response [11], the ZUMA-2 study's findings revealed that the median tumor burden (SPD) for ongoing responders and relapsed responders was 935.1 and 4233.6 mm2, respectively [12]. In the ZUMA-2 study, the ongoing responders and relapsed responders shared a similar incidence of high-risk features (a baseline Ki-67 proliferation index score, elevated lactose dehydrogenase levels and simplified MIPI scores). Despite the fact that CR rates are commonly comparable, patients with high tumor burden are at risk of lymphoma relapse with a short OS [13]. In this case report, our patient had a much larger SPD (19124 mm2) than those who had no durable responses to KTE-X19, while achieved prolonged remission, which lasted for over 2 years and defied the high relapse risk of high tumor burdens.
The predictive power of high tumor burden for the incidence and severity of CRS [14] and ICANS [15] has been well established. Because T-cells engineered with 4-1BB co-stimulation expand more gradually and have longer durability than CD28-costimulated products, CARs incorporating 4-1BB exhibit lower incidence rates of CRS and ICANS compared to approved CD19 CAR-T products [16,17]. The patient was given relma-cel, a second generation of CD19 CAR-T with a 4-1BB costimulatory domain. This type of CAR-T has been demonstrated to have lower rates of toxicities, with 5.1% of LBCL patients experiencing grade ≥ 3 CRS and grade 3 ICANS [18], no FL patients experiencing grade ≥ 3 CRS, and 3.6% of FL patients experiencing ICANS [19]. Severe and refractory CRS and ICANS were much more common in patients with highly proliferative aggressive diseases, such as r/r MCL, especially the heavily pre-treated patients when they were treated with CAR-T cell infusion [20]. Furthermore, there was a greater chance that patients with severe CRS and ICANS would fail the CAR-T therapy [21]. After failing 7 lines of systemic therapies, our patient with high tumor burden experienced only grade 2 CRS while recovered within 8 days. He achieved CR for more than 2 years. These observations demonstrated the durable response and safety profile with relma-cel in our patient.
Toxicities of CAR-T cells have been frequently associated with higher peak levels of cytokine and CAR-T cells expenson [22-25]. Patients with higher tumor burden showed increased CAR-T expansion and toxicity [23]. According to the conclusion by Wang et al. in the ZUMA-2 study, higher expansion patients had a deeper response and a higher incidence of severe CRS. Peak CAR-T cells expansion level was higher in responders (median peak of 102.4 cells/μL) as compared with the nonresponding patients (median peak of 12.0 cells/μL) [2]. Nevertheless, no correlation between expansion and baseline tumor burden was found [10,26]. In the patient in our study, a peak level of CAR-T cell expansion was determined to be 1766.0 cells/μL by flow cytometry at Day 11 after infusion and 351337 copies/μg by qPCR at Day 15 after infusion, demonstrating that the patient, who achieved a long-term response, had a higher degree of relma-cel expansion and lower toxicities with only grade 2 CRS.
There is currently another mechanism that relates to the serum cytokine levels and underlies toxicities and responses with CAR-T cells. The primary cause of many core symptoms of severe CRS (grade ≥ 3) was assumed to be IFN-γ and IL-6 cytokines [27], showing the significance of the cytokines in the clinical setting [28,29]. In our study, despite the highest levels of IL-6 (beyond 3x ULN) and IFN-γ (up to 5x ULN), the patient's exceptional performance with regard to toxicities with lower incidence of CRS and NT have contributed to his long-term response to the relma-cel therapy.
In conclusion, this case report suggests that relma-cel elicited a potent and durable anticancer response and good tolerance with no safety concern in a patient with high tumor burden despite a tough clinical challenge associated with the systemic treatment with r/r MCL. Additional information about relma-cel in r/r MCL will be published in the phase II study (NCT04718883).
Thanks for the provided funding from JW Therapeutics (Shanghai) Co. Ltd.
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
Jun Zhu: Project administration, data acquisition, and article review.
Yuqin Song: Data acquisition, statistical analysis, blood sample collection, patient evaluation, and article review.
Xie Yan: Data acquisition, patient evaluation, and article preparation.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Xie Y, et al. "Relma-cel Elicited Durable Remission in a r/r MCL Patient with High-Risk Factors: A Case Report". Oncol Cancer Case Rep, 2024, 10(3), 1-5.
Received: 19-Apr-2024, Manuscript No. OCCRS-23-132596; Editor assigned: 22-Apr-2024, Pre QC No. OCCRS-23-132596 (PQ); Reviewed: 07-May-2024, QC No. OCCRS-23-132596; Revised: 19-Jun-2024, Manuscript No. OCCRS-23-132596 (R); Published: 27-Jun-2024, DOI: 10.35248/2471-8556.24.10(1).003
Copyright: © 2024 Zhu J. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.