Research Article - (2016) Volume 7, Issue 1
Objective: The purpose of the study was to quantitatively measure the amount of physical activity during standardized activities using accelerometers in patients with transient ischemic attack (TIA) in order to compare different physical activities based on objective measurements.
Methods: Thirteen hospitalized TIA patients performed 11 different standardized activities with 5 Actical accelerometers attached to both wrists and ankles and the hip. The output was registered as activity counts (AC). Total AC were the sum of AC from all five accelerometers.
Results: Higher total AC/minute were found in more intensive activities, e.g. the median total AC/minute were 11216 (IQR 8447-14153) during climbing stairs and 22 (IQR 12-42) during sleep.
Conclusion: The study demonstrates that it is possible to compare different physical activities based on amounts of AC/minute obtained during standardized activities in TIA patients. Accelerometer outputs recorded during standardized physical activities enable future tailoring of specific rehabilitation programs for individual stroke patients aiming at a certain amount of AC by combining different specific physical activities.
Keywords: Stroke; Exercise; Rehabilitation; TIA; Accelerometry; Standardized activity
The use of accelerometers in research has increased exponentially in the recent years [1], also within the field of stroke, where the effects of mobilization in stroke patients are still debated and under investigation [2]. However, the interpretation of accelerometer output, the activity count, is difficult and activity counts (AC) are not easily comparable between different accelerometer makes [1,3,4]. In previous studies activity intensity thresholds have been established to categorize activities measured with accelerometers as either light, moderate or vigorous for specific makes, among others the Actical accelerometer used in this study [5–9]. Intensity cut-points are based on AC/minute for particular populations, often younger healthy adults, and are not currently available for stroke patients or the Actical accelerometer.
Another approach in aiding AC interpretation is by direct comparison of AC from different studies of comparable populations measured with the same accelerometer make. However, studies employing such an approach are as yet rare and a reference material comprising AC from different populations, which will be needed to facilitate direct comparison of physical activities reported in different studies, is not available.
The purpose of the present study was to provide AC from standardized physical activities in order to enable future tailoring of rehabilitation programs based on quantitative and objective measurements. The amount and distribution of physical activity were measured with 5 accelerometers in 11 standardized activities, such as turning in bed, getting up from a chair, walking and climbing stairs in patients with transient ischemic attack (TIA) admitted to the comprehensive stroke unit at Copenhagen University Hospital- Nordsjællands Hospital, Hillerød, Denmark.
Study population
The study was an open, descriptive study approved by the Regional Ethics Committee (no. H-1-2012-001). Written informed consent was obtained from the participating patients.
Patients with a diagnosis of TIA and age >18 years were included from the acute stroke unit at Copenhagen University Hospital– Nordsjællands Hospital, Hillerød, Denmark. The study period was June 2012 to December 2013. Patients were included only if they scored normal values on Scandinavian Stroke Scale (SSS; range 0-58, a score of 58 indicates no neurological deficits) [10], National Institutes of Health Stroke Scale (NIHSS; range 0-42, a score of 0 indicates no neurological deficits) [11], Barthel Index 100 (BI; range 0-100, a score of 100 indicates no deficits in performance of activities of daily living) [12] and modified Rankin Scale (mRS) [13], i.e., had no neurological or functional deficits. Patients were excluded if 1) informed consent was not given within 4 hours of receiving the project information or 2) the standardized activities could not be completed within 2 days.
Accelerometers
Physical activity was measured with Actical accelerometers (Philips Respironics), which are small (29 × 37 × 11 mm and 16 g) omnidirectional, waterproof, piezoelectric accelerometers. Accelerometers are motion sensors which also include ‘pedometers’ that measure number of steps during gait [14]. Other frequently used accelerometer makes are ActiGraph, Caltrac, RT3 and TriTrac [1].
Actical accelerometers register vibrations during acceleration, which produce a proportional variable electrical voltage [15]. The frequency range is 0.5-3.9 Hz with a sampling frequency of 32 Hz [16]. The accelerometer output is expressed as AC in epochs of 15 seconds. Total AC indicate AC summed for all 5 accelerometers.
A previous reliability study tested Actical accelerometers during 6 different hydraulic shaker table conditions. The intrainstrument reliability estimated as the mean coefficient of variation was found to be 0.4% to 0.5% and the mean coefficient of variation for the interinstrument reliability was 5.4% to 15.5% [17].
Patients were included on the day of their discharge and were tested with SSS, NIHSS, BI and mRS. Patients were asked to perform 11 different activities (see below) with pauses in between. Before the start of the first activity, Actical accelerometers were attached to both wrists and both ankles using Velcro bands or plastic straps. One accelerometer was placed over the right anterior superior iliac spine by means of an elastic belt. Each activity was marked by pressing each accelerometer’s event button just prior to the start and after completion of the activity. Duration of the activity was also recorded with a stopwatch. Having completed all activities except “sleeping,” patients were instructed how to attach the accelerometers in the correct way. The accelerometers were marked with the name of the correct placement, e.g. right arm. Each accelerometer was worn at the same anatomical placement throughout all activities. Patients were instructed to attach all 5 accelerometers at home prior to sleep and mark their sleep by pressing the event button of at least one accelerometer at the time they went to bed and immediately after waking up. The duration of sleep was calculated backwards from the time of awakening.
The standardized activities were: 1) in a sitting position, lifting a glass of water placed 50 cm from the patient’s torso approximately at the level of the umbilicus, drinking the water and putting the glass down again; 2) right shoulder flexion from 0 to 90° in a standing position × 5; 3) 5 minutes of eating while sitting; 4) turning in bed from left to right side and back again; 5) rising from a chair and sitting down again × 5; 6) changing position from lying supine in bed to standing next to the bed; 7) climbing stairs one floor up and down (22 steps of 17 cm) again at a comfortable pace; 8) cycling on an exercise bike without resistance for 5 minutes; 9) walking on a treadmill for 5 minutes at 0.28 m/s; 10) walking on a treadmill for 5 minutes at 0.56 m/s; 11) sleeping for 6 hours at home.
Statistical Analysis
Data were described using means and standard deviations for normally distributed variables. Accelerometer data are reported using medians and interquartile ranges (IQR).
Missing data (MD) were defined as 1) clearly erroneous recording or erroneously missing recording, i.e., incorrect zero output on one or several accelerometers, 2) non-wear time registered by the investigator or 3) a recording error for other reasons. Accelerometer outputs with MD were excluded from the analysis only when relevant. MD resulted in a varying number of patients for the different standardized activities.
| Characteristic | n | |
|---|---|---|
| Age, mean years ±SD | 68.8 ±7.5 | 13 |
| Gender, % male/female | 31/69 | 13 |
| Body Mass Index, mean kg/m2 ±SD | 25.8 ±4.1 | 11 |
Table 1: Demographic data for included patients (SD: Standard Deviation; IQR: Interquartile Range).
Figure 1: The distribution of total AC/minute during 11 different activities, series 2, shown as a box and whiskers plot (n=12, 1 patient excluded due to MD; for activity 11: n=10, 1 patient excluded due to MD and 2 patients excluded due to lack of recording). The length of the box represents the interquartile range with the group median shown as an interior horizontal line. The whiskers represent values from the upper or lower boundary of the box plus or minus 1.5 × the interquartile range with outliers outside this range represented by circles. Detailed description of activities is explained in the article.
Study population
16 patients with an initial working diagnosis of TIA were included in the study during the 18 month enrolment period. 3 patients were excluded due to change in diagnosis after inclusion resulting in 13 patients completing the study. Demographic data are presented in Table 1. Only 11 patients wore accelerometers during sleep (activity 11). Two patients slept less than the set 6 hours as per protocol but the available data from these two patients were included in the analysis.
Accelerometer data
MD were found in 1.0% of the total file time for all patients measured during the 11 activities, and resulted from one accelerometer in one patient measuring clearly erroneously high values during recording.
Standardized activities in TIA patients
Total AC/minute was higher in more intensive activities such as climbing stairs, cycling and walking on treadmill at a higher pace (Figure 1). Some activities had very small AC/minute when AC/minute was calculated as medians and IQR for individual accelerometers. For example, the calculated median AC/minute in activity 3 (eating while sitting) was less than ten for the legs and the hip and 100-150 AC/ minute for each arm (Table 2).
| Median AC/minute (IQR) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Activity | Right arm | Left arm | Right leg | Left leg | Hip | Total | n | |
| 1. drink a glass of water | 108.0a (31.0-492.0) |
0.0 (0.0-5.0) |
0.0 (0.0-0.0) |
0.0 (0.0-0.0) |
0.0 (0.0-0.0) |
177.0 (31.0-569.0) |
12c | |
| 2. right shoulder flexion from 0 to 90° while standing up, ´ 5 | 976.0 (0.0-1474.0) |
0.0 (0.0-15.0) |
0.0 (0.0-0.0) |
0.0 (0.0-0.0) |
0.0 (0.0-0.0) |
1099.0 (10.0-1592.0) |
12c | |
| 3. eat while sittingb | 142.2 (136.1-305.3) |
100.7 (49.3-244.6) |
5.1 (1.0-118.5) |
3.0 (0.5-154.9) |
4.0 (0.0-62.7) |
271.7 (196.8-853.5) |
12c | |
| 4. turn in bed from left to right side and back again | 988.0 (0.0-1200.0) |
1030.0 (938.0-1146.0) |
99.0 (0.0-512.0) |
32.0 (0.0-406.0) |
268.0 (38.0-474.0) |
2726.0 (1576.0-3372.0) |
12c | |
| 5. rise from chair and sit down again, ´ 5 | 700.0 (195.0-1194.0) |
578.0 (117.0-1144.0) |
21.0 (0.0-155.0) |
30.0 (0.0-234.0) |
326.0 (38.0-542.0) |
1758.0 (1249.0-3076.0) |
12c | |
| 6. standing up from supine position in bed to a standing position next to the bed | 0.0 (0.0-128.0) |
0.0 (0.0-0.0) |
0.0 (0.0-0.0) |
0.0 (0.0-60.0) |
0.0 (0.0-60.0) |
102.0 (0.0-992.0) |
12c | |
| 7. climbing up and down stairs, 1 floor at comfortable pace | 1765.0 (1336.3-2402.0) |
1309.0 (942.0-1564.0) |
3654.0 (2192.0-4561.0) |
3599.0 (2317.3-4413.0) |
1056.7 (839.0-1617.0) |
11216.0 (8447.0-14153.0) |
12c | |
| 8. cycling on exercise bike† | 15.1 (4.6-57.8) |
11.0 (0.0-41.7) |
4221.4 (3286.6-5654.9) |
4258.9 (3513.9-5411.6) |
1.0 (0.0-33.4) |
8511.3 (6856.5-11400.9) |
12c | |
| 9. treadmill walking at 0.28 m/s† | 35.2 (22.5-106.1) |
34.3 (4.9-158.6) |
464.0 (360.6-910.5) |
642.9 (349.2-979.4) |
1.0 (0.0-13.5) |
1281.6 (718.9-2171.1) |
12c | |
| 10. treadmill walking at 0.56 m/s† |
161.4 (18.3-341.6) |
47.0 (1.0-307.1) |
1556.1 (1253.1-1913.6) |
1666.5 (1077.2-2049.6) |
203.0 (134.3-341.4) |
3510.4 (2808.9-4770.5) |
12c | |
| 11. sleeping | 8.6 (4.0-12.5) | 6.0 (4.7-12.6) | 3.0 (1.3-4.7) | 2.5 (0.9-7.3) | 1.0 (0.5-4.3) | 22.1(12.2-41.6) | 10cd | |
Table 2: Median activity counts (AC) per minute for standardized activities. (aResult presented as AC for the arm used for the activity. bBased on 5minute observation time. c1 patient excluded due to MD. d2 patients excluded due to lack of recording. IQR, interquartile range).
An Example of a Case with Patient Specific Tailoring of Rehabilitation
A 61-year old female admitted with an ischemic stroke and a moderate right hemiparesis (SSS 41, NIHSS 9, BI 70 and mRS 3). During the first 24 hours after admission the patient has a total of 193175 AC (sum of all five accelerometers).
As part of the rehabilitation the patient is encouraged to achieve the same amount of total AC by other activities and thus double the 24- hour activity level. The patient is only able to walk slowly on a treadmill for a short period of time but can cycle on an exercise bike for a longer period. Doubling of the total AC in the rehabilitation programme can be achieved by adding:
10 minutes of slow treadmill walking (0.28 m/s, cf. Table 2):
10 minutes × 1281.6 AC/minute=12816 AC
and
21 minutes of cycling on an exercise bike (cf. Table 2):
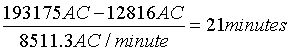
On day 5 after admission the patient walks on a treadmill for 30 minutes with a total of 25023 AC for the paretic right leg and is about to be discharged.
To continue with the current activity level at home further rehabilitation may consist of:
6 minutes of cycling on an exercise bike (cf. Table 2):
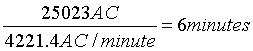
or
7 minutes of climbing stairs (cf. Table 2):
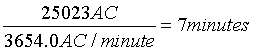
This study reports AC/minute for 11 different standardized activities and shows higher total AC/minute in high intensity activities such as cycling, walking on a treadmill or up and down stairs. The study is the first to measure AC/minute of selected standardized activities that are systematically performed by patients hospitalized for TIA.
As expected, lower AC/minute were found in low intensity activities with median 0 AC/minute measured by individual accelerometers during some of these activities. In other studies low activities of 0 or 1 AC/minute have been measured over the hip [7–9,18] and over the ankle [8] in healthy individuals during supine resting, TV viewing and card sorting. Higher AC/minute, particularly for the arms and the hip, were registered in activity 5 (rise from chair and sit down again × 5) compared to activity 6 (standing up from supine position) in which 0 AC/minute were registered. The explanation for this observation may be the number of repetitions of the activity. Thus, the vertical axis was involved nine times more for activity 5 than 6, although the latter activity seems more complex. On the other hand, relatively high AC/ minute were measured in activity 4 (turning in bed from left to right side and back again). Accelerometers measure acceleration and will thus register AC only with changes in speed. It could therefore be speculated that the change in movement (left to right etc.) gives higher AC, as compared to forward movement (up, forward, etc.), due to greater and more frequent changes in speed.
Higher intensity activities showed a greater disparity in AC/minute between the current and previous studies, which may be due to differences in the populations. For instance, AC/minute measured over the hip during stair climbing in an older population was similar to the current study [7]. However, physical activity during cycling was only median 1 AC/minute over the hip in the current study versus 157 AC/ minute found previously where the participants were on average 35 years old [9]. For climbing stairs the same study measured more than double the median AC/minute compared with the current study.
Similarly, higher treadmill speeds in other studies make a direct comparison with the current results difficult [6–8]. Previously, we have measured physical activity in acute ischemic stroke patients with a leg paresis during treadmill training and the current AC/minute during treadmill training at a speed of 0.56 m/s are similar to the measured AC/minute after 5 days of treadmill training at a speed of 0.5 m/s [19]. This indicates that standardized activities can be compared in similar populations over more intensive and longer activities. For patients who have received treadmill training during hospitalization, it might be relevant to encourage the patients to continue training after discharge. To achieve the same amount of physical activity it is possible to calculate an equivalent physical activity dose for other easy and more available activities, such as stair climbing or cycling. For instance, based on the findings of our previous study, the AC from a leg registered during 30 minutes of treadmill training on day 30 after inclusion, can be achieved by 19.9 minutes of climbing stairs (activity 7) or 17.0 minutes of cycling on an exercise bike (activity 8) [19]. Previously we have also continuously measured AC in both acute ischemic stroke and TIA patients for periods of 24 hours [20], and found that total AC in the first 24 hours after inclusion were 195,868 and 681,078, respectively. To achieve the same amount of total AC, acute ischemic stroke patients could instead cycle for 23 minutes or climb stairs for 17 minutes daily. In TIA patients, the corresponding duration of physical activity is 80 minutes of cycling or 61 minutes of climbing stairs. Thus, the results show that for ischemic stroke patients, the 24-hour level of physical activity can be increased significantly by adding basic physical activities for limited periods of time during hospitalization in an acute stroke unit. An example of how a rehabilitation program might be tailored in an individual patient based on AC/minute registered during standardized activities is shown in the box.
The study was conducted in TIA patients who are similar to ischemic stroke patients in terms of risk factors and symptoms at onset. In addition, the AC/minute-levels during standardized activities observed in the current study are indicative of what might be expected in stroke patients without disabilities. The standardized activities in the study are representative for activities occurring during hospitalization. A larger number of patients would have been strength, but unfortunately inclusion of hospitalized patients prior to discharge limited the number of patients willing to participate.
In conclusion, the study reports quantitative data from standardized activities during hospitalization after TIA and demonstrates that accelerometers are applicable in the planning of rehabilitation programs for stroke patients. It is thus possible to compare different physical activities based on amounts of AC/minute obtained during standardized activities in TIA patients. Based on these findings it will be possible to tailor a specific rehabilitation program for the individual stroke patient aiming at a certain amount of AC by combining different physical activities. With such a personalized rehabilitation program it will be possible to target a predefined increase in overall physical activity or activity of individual extremities in stroke patients by combining different activities such as cycling, climbing stairs or walking. Some low intensity activities showed very low median and IQR AC/minute in individual accelerometers, which indicates that accelerometers should be used to measure AC over longer lasting activities. AC/minute were in general very low in the hip accelerometers, and for future studies in stroke or TIA patients we would recommend the use of accelerometers over extremities, primarily the ankle, rather than the hip.
Anna M Strømmen was supported with a research grant from Copenhagen University Hospital–Nordsjællands Hospital, Hillerød, Denmark and funding from Department of Neurology, Copenhagen University Hospital – Nordsjællands Hospital, Hillerød, Denmark and Olga Bryde Nielsens Foundation. The authors wish to thank Department of Public Health, Section of Biostatistics, University of Copenhagen for statistical support prior to and during the study. Finally, we would like to thank all the patients who agreed to participate in the study.