Case Report - (2024) Volume 10, Issue 1
Background: A minority (4%-15%) of Gastrointestinal Stromal Tumours (GISTs) lack common gene mutations and undergo dedifferentiation, either de novo or following chronic imatinib treatment, exhibiting remarkable histological and immunophenotypic changes and possible heterologous differentiation. Given the lack of the usual immunophenotype and molecular signature, dedifferentiated GISTs represent a diagnostic challenge and potential pitfall with no concrete guidelines for its management and associated poor prognosis. In our patient’s case, molecular testing also detected a rare somatic TP53 mutation, making it an extremely rare form of an already rare tumour.
Aim: To illustrate dedifferentiated GIST as a potential diagnostic pitfall and clinical challenge through the case of a 41-year-old, imatinib-naïve male who presented with upper gastrointestinal bleeding and was subsequently diagnosed with dedifferentiated GIST.
Methods: The dedifferentiated component showed a loss of CD117 and DOG-1 expression that was otherwise present in the conventional component, thus confirming the diagnosis. Further immunohistochemistry excluded other high-grade spindle cell neoplasms, and molecular testing confirmed our diagnosis.
Results and Conclusion: This case illustrated the necessity of sufficient sampling to successfully identify and diagnose a dedifferentiated GIST, and the importance of considering dedifferentiated GIST as a differential diagnosis when high-grade spindle cell neoplasms occur in the gastrointestinal tract as they can be erroneously misdiagnosed due to their heterogenous morphology, lack of CD117 and DOG-1 staining, and rarity. This case also demonstrated the need for concrete guidelines and further research into the clinical presentations, diagnostic and management pathways to improve patients’ quality of life, treatment success and prognosis.
Gastrointestinal stromal tumours • Immunophenotype • Gastrointestinal tract
We present a unique case of a dedifferentiated Gastrointestinal Stromal Tumour (GIST) arising de novo in a 41-year-old man with no prior history of tyrosine kinase therapy. Dedifferentiated GISTs have a high-grade sarcoma component and lack the common mutations, histomorphology and immunophenotype associated with GISTs. Clinically, differentiated GISTs are a diagnostic challenge with no concrete guidelines to its management and associated poor prognosis. In our patient’s case, molecular testing also detected a rare somatic TP53 mutation, making it an extremely rare form of an already rare tumour.
A 41-year-old gentleman with known pulmonary sarcoidosis, hypertension and legal blindness resulting from a history of pilocytic astrocytoma. He presented with coffee ground vomitus and dark-coloured stools, consistent with an upper gastrointestinal haemorrhage. Of significance, the patient had no prior history of tyrosine kinase therapy. He underwent investigations as outlined below.
The preoperative blood showed a significant haemoglobin drop to 36g/L, requiring transfusion with 5 units of packed red blood cells and 2 units of fresh frozen plasma. The patient underwent a staging CT scan which demonstrated a lobulated hypo-enhancing gastric body mass and gastroscopy revealed a polypoid greater curvature lesion with central ulceration. Biopsy showed a high-grade sarcoma which was CD117 and DOG1 negative and desmin, WT1 and SMA positive. This led to a diagnosis of a malignant spindle cell tumour with smooth muscle differentiation. Subsequent laparoscopic sleeve-type gastrectomy revealed a polypoid, fungating mass on the greater curvature.
The macroscopic review showed a well-circumscribed, encapsulated, nodular lesion with partial mucosal ulceration and haemorrhagic foci, measuring 90x65x55mm and weighing 156g. After extensive sampling, a high-grade, predominantly spindle cell neoplasm with a plexiform growth pattern and two distinct cytomorphological appearances were identified (Fig 1A). The lower-grade area was composed of monotonous spindled cells arranged in fascicles with eosinophilic, fibrillary cytoplasm and indistinct cell borders. This transitioned to a dedifferentiated morphology featuring heterologous differentiation with cellular proliferation of highly pleomorphic and multinucleated spindled and epithelioid tumour cells, high mitotic activity (>50/50HPF) and scattered atypical mitoses (Figure 1A-B).
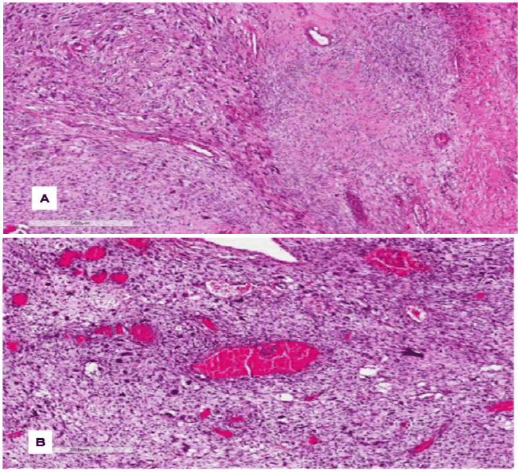
Figure 1: (A) H&E image â?? low power field showing tumour with transition from low to high-grade components. (B) H&E image â?? high power field showing high-grade anaplastic features.
Immunohistochemistry showed patchy positive immunoreactivity for CD117 desmin, WT1, SMA and beta-catenin in the lower-grade area, which was otherwise lost in the adjacent dedifferentiated area (Figure 2A), DOG1 (Figure 2B). Melanoma and pan-keratin markers were negative. Other immunohistochemistry stains including SS18-SSX, NUT, MDM2 and SDHB were also negative.
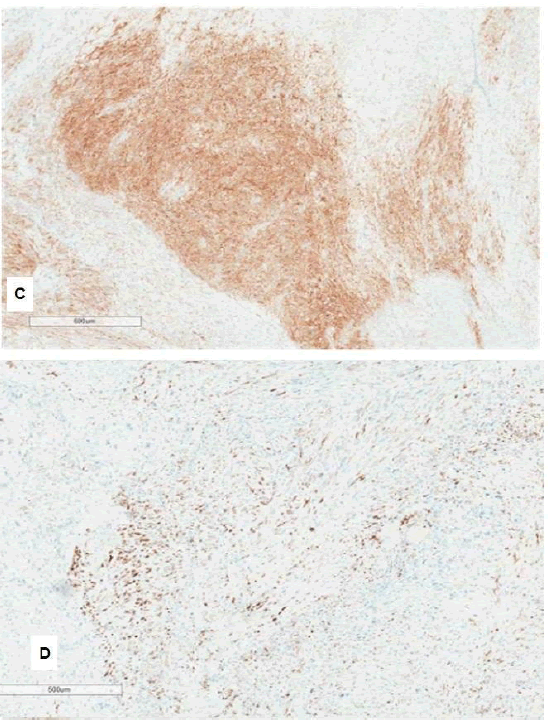
Figure 2: (A) Immunostain for CD117 showing patchy positive staining (B) Immunostain for DOG1 showing patchy positive staining
Given the site, histomorphology and immunophenotype of the lower-grade areas with patchy CD117 and DOG1 positivity, our provisional diagnosis was dedifferentiated GIST. Subsequent molecular testing detected somatic TP53 mutation and a decision was made to trial adjuvant imatinib therapy.
Unfortunately, our patient developed poor appetite and abdominal pain five months after commencing imatinib therapy. Subsequent repeat CT scan showed rapid intra-abdominal disease recurrence with a newly developed large, lobulated and necrotic soft tissue mass (125x117x145 mm) arising along the previous staple line of the greater curvature of the stomach, partially compressing the gastric lumen. Surrounding irregular and nodular stranding infiltrates suspicious for local spread with metastatic infiltration, para-aortic and aortocaval nodal disease and evolving lung metastases were also present. Given the size and diffuse infiltration of the tumour, radiotherapy and surgical options were deemed unlikely to be beneficial and the patient ceased imatinib therapy and was commenced on palliative doxorubicin chemotherapy.
After completing three cycles of doxorubicin, the patient re-presented with febrile neutropenia, vomiting, abdominal pain and recurrent rectal bleeding. A repeat CT scan demonstrated an increase in the size of the known lobulated, necrotic tumour (221x151x228mm), exerting a significant local mass effect onto the stomach which was markedly compressed. After extensive discussion, the decision for debulking surgery was made to provide symptomatic improvement by relieving local mass effects and direct tumour invasion into the surrounding bowel. Intraoperatively, the tumour was found to be cystic and necrotic, containing four litres of haemopurulent material.
Regrettably, five months later, a follow-up CT scan then demonstrated two new lobulated hypodense lesions, with one lesion closely related to the greater curvature of the stomach measuring 75x72x27mm and one lesion in the epigastric region measuring 40x55x74mm. Less than one month later, the patient was admitted to the hospital with a minor right hemispheric subcortical ischaemic stroke and pain crisis associated with his tumour burden. His stroke was managed conservatively, however, repeat CT scans performed one week apart demonstrated significant enlargement of the two gastric lesions (118x63x143mm and 124x104x244mm) which then formed a large conglomerate mass measuring 275x129x322mm, causing local mass effect and partial encasement of small bowel loops with risk of obstruction. Subsequently, the patient underwent a debulking laparotomy and adhesiolysis, revealing an intrabdominal tumour occupying most of the abdomen. During his most recent follow-up, he was referred to community palliative care for future ongoing management.
GISTs are the most common mesenchymal neoplasms of the Gastrointestinal Tract (GIT) and exhibit monotonous morphology, minimal cytological atypia and rare mitoses [1-3]. They characteristically express CD117 (KIT oncoprotein) and DOG1 immunoreactivity, which has come to define the pathologic diagnosis of GISTs [3-5].
GISTs arise from the interstitial cells of Cajal, which function as pacemakers for GIT peristalsis, and CD117 is a receptor tyrosine kinase crucial in the differentiation and growth of these cells. When CD117 is mutated to become constitutionally active, tumorigenesis of GIST occurs. This predictable pathogenesis allows CD117 immunohistochemistry to be highly specific and sensitive for GIST. Around 5% of GISTs are wild-type for KIT and are diagnosed by DOG1 or molecular testing. The median age of diagnosis is 60 years old, with no gender predilection. Paediatric GISTs are considered a separate pathologic entity with cases peaking around 10-20 years of age [6].
In general, the majority of GISTs show activating mutations in either the KIT or PDGFRA proto-oncogenes, and less commonly in SDH and BRAF.3-5 However, a minority (4%-15%) of GISTs lack these mutations and present as dedifferentiated GISTs.1,5 In our case, although molecular testing did not identify C-KIT, PDGFRA or BRAF gene mutations, somatic TP53 mutation was detected. Such TP53 variants are rarely described in GISTs and be associated with sarcomatous histomorphology and overall poor survival [7]. Clinically, differentiated GISTs are a diagnostic challenge with no concrete guidelines for their management and associated poor prognosis.
Dedifferentiated GISTs also exhibit histomorphological and immunophenotypic changes, including increased cellularity with pleomorphism, marked nuclear atypia, high mitotic activity, necrosis, CD117 and DOG1 immunoreactivity, and possible heterologous differentiation [2,8]. Historically, dedifferentiated GISTs have been reported to arise in patients with a history of tyrosine kinase inhibitor treatment and speculated to occur as part of a morphological tumour progression from a conventional CD117- positive GIST to a dedifferentiated CD117-negative GIST due to a KITinduced mechanism of resistance to chronic tyrosine kinase therapy [2,9]. However, in recent years, de novo dedifferentiated GISTs have emerged as a newly identified entity, as was seen in our case. Given the lack of the usual immunophenotype and molecular signature, dedifferentiated GISTs represent a diagnostic challenge and potential pitfall. This case demonstrated the importance of considering dedifferentiated GIST as a differential diagnosis when high-grade spindle cell neoplasms or an undifferentiated sarcoma component is identified in the GIT, as they can be erroneously misdiagnosed. As such, additional immunohistochemical and molecular investigations are necessary to help exclude other potential mimics [3, 4]. This case also illustrated the necessity of sufficient sampling to successfully identify and diagnose a dedifferentiated GIST.
In summary, we expand upon the existing literature on dedifferentiated GISTs arising de novo without prior prolonged tyrosine kinase exposure and present an extremely rare case of an already rare tumour that demonstrated heterologous differentiation, with a distinct delineation between the two tumour morphologies consistent with conventional and dedifferentiated GIST and an unusual somatic TP53 mutation. It is important to consider dedifferentiated GIST as a differential diagnosis when high-grade spindle cell neoplasms occur in the GIT, as they can be erroneously misdiagnosed due to their heterogenous morphology, lack of CD117 and DOG-1 staining, and rarity.
Citation: Xing H., et al. Unique Case Report of a Rare Form of Dedifferentiated Gastrointestinal Stromal Tumour. Oncol Cancer Case Rep. 2024, 10(01), 001-003.
Received: 07-Dec-2023, Manuscript No. OCCRS-23-122264; Editor assigned: 11-Dec-2023, Pre QC No. OCCRS-23-122264 (PQ); Reviewed: 02-Jan-2024, QC No. OCCRS-24-122264 (Q); Revised: 08-Jan-2024, Manuscript No. OCCRS-24-122264 (R); Published: 16-Jan-2024
Copyright: ©2024 Xing, H. This is an open-access article distributed under the terms of the Creative Commons Attribution License CC-BY, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.