Research Article - (2022) Volume 7, Issue 10
Oncological patients need the proper doses of medications to facilitate their recovery. The two basic approaches used in dosing Monoclonal Antibodies (mAbs) are fixed-dose combination and variable dosing. In Fixed-Dose Combination Drugs (FDCs), two or more active components are combined in a single formulation at a predetermined dose. Variable dosage, which has long been the industry standard, is the polar opposite of this approach. The body changes over time; the Body Surface Area (BSA) in square meters is often used as a Measure (m2). This study uses a systematic review. Most mAbs used in oncology are predominantly given as cytotoxic anticancer drugs using body-size-based (variable) regimens. Despite the benefits of fixed-dose, variable dosing has become the industry standard, despite being criticized for ineffectiveness. While variable dosing has some advantages, the prevalent view is that continuous dosing has significant advantages based on the balance of probabilities. After assessing each alternative, including its benefits and drawbacks, history of use, and suitability in the current context, fixed dosing emerges as a viable option.
Monoclonal antibodies • Variable dosing • Fixed dosing • Oncology
Background of the research
Monoclonal Antibodies (MAbs) have varied applications, but they are most significant in oncology. MAbs have been approved for over 30 diseases, with the most common applications in cancer [1]. Before delving into the dosing of MAbs, it is worth comprehending their origin and development. Lu et al. (2020) opine that B Cells and specifically target antigens are used in the production of MAbs. However, their massive production is facilitated by the hybridoma technique that Milstein and Kohler introduced in 1975 [2]. The ultimate approval of MAbs paved the way for their application to oncology and other fields. Lu et al. (2020) note that the United States Food and Drugs Administration approved the first mAbs in 1986, consequently paving the way for their application and evolution in technological development. Therefore, mAbs have had an extended usage period in oncology, especially in cancer treatment.
Effective dosing of MAbs is critical for cancer patients. MAbs are administered through body-sized dosing (variable dosing) or fixed dosing in oncology. The choice of dosing depends on the oncologist and the desired outcomes for the patient [3]. However, other researchers, reiterate that the most preferred dosing approach has been body-sized dosing (variable dosing) in contemporary oncological practice [4]. The primary justification for using variable dosing of MAbs in the current oncological practice is a need to correct the variability in drug distribution and elimination among patients that leads to the desired patient outcomes [4]. Fixed dosing is less efficient in correcting drug variability related to the distribution and elimination of the drug.
Research problem
MAbs play a critical role in the treatment of cancer. However, the biggest problem in the contemporary practice of oncology is the determination of the correct and most effective approach to dosing. The most significant debate is whether to adopt body-sized dosing (variable dosing) or fixed dosing for patients. The argument that has pushed for the continued application of body-sized dosing has been driven by the perception that the approach reduces variability in drug exposure among users [5]. The meaning here is that when individuals become leaner or heavier in terms of their weight, they will experience variability in drug exposure if the same dosages are used. Nevertheless, Bai et al. (2012) argue that most MAbs are target-specific and have a relatively extensive therapeutic window, and they have a minimal contribution to the variability in drug exposure among different patients. Hendrikx et al. (2017) reiterate that fixed dosing is recommended mainly in cases with the minimum effect of the body weight on the volume of distribution and clearance. However, if the impact of the variability is strong or unknown, there is always a recommendation to utilize variable dosing [4]. Therefore, the problem in the current oncological practice is to determine the best possible approach to dosing that can ensure MAbs efficiency while also limiting potential diverse effects on patients.
Significance of the research
The significance of this research is based on the divided opinions or debates around variable vs. fixed dosing of MAbs. The research is significant because it will explain their efficacy and effects in treating cancer patients in-depth. Besides, there will be an in-depth exploration of the pros and cons of variable (body-sized dosing) and fixed dosing in terms of the pharmacokinetic variability they cause. This will lead to determining the most effective dosing approach. Bai et al. (2012) explain that the argument that has pushed for the continued application of bodysized dosing has been driven by the perception that the approach reduces variability in drug exposure among users based on the best possible outcomes for cancer patients. Therefore, this study is significant because it assesses both the variable dosing regimen and the fixed dosing regimen comparing their advantages and disadvantages. The most effective dosing approach can be selected based on the disadvantages and advantages.
Research aim and objectives
The research compares MAbs' variable vs. fixed dosing, highlighting their pros and cons and determining the most effective dosing approach.
The objectives of the study are as follows:
• To examine variable dosing (body-size dosing) of MAbs in oncology, highlighting its pros and cons.
• To explore fixed dosing of MAbs in oncology highlighting its pros and cons.
• To propose an effective dosing approach based on the pros and cons of variable dosing and fixed dosing of MAbs in oncology.
Research questions
The research questions that this study answers are as follows:
• What are MAbs' variable dosing (body-size dosing) in oncology, and what are its pros and cons?
• What is fixed dosing of MAbs in oncology, and what are its pros and cons?
• What is the most effective approach to dosing MAbs in oncology based on the pros and cons of variable dosing (body-size dosing) and fixed dosing?
This chapter discusses previous studies presented on the topic. However, the first part of this chapter focuses on the theoretical framework related to dosing and determining the best possible dosing regimen for cancer patients. Oncologists have to determine a dosing regimen that ensures maximum recovery outcomes while mitigating adverse outcomes to attain maximum outcomes. The review focuses on optimal control theory and other studies illustrating varied and fixed dosing of monoclonal antibodies in oncology.
Theoretical framework
Optimal control theory: The optimal control theory is the leading theory used in this research to evaluate the dosing regimen applied to cancer patients. The optimal control theory is a branch of mathematics. The establishment of an effective dosing regimen is considered a dynamic system [6]. Applying the optimal control theory to the design of personalized cancer treatments by noting that most clinicians are still grappling with establishing successful personalized therapies [7]. Hence, there is a need to determine a reliable approach to predicting drug dosing and the impact that it will have on a patient.
The choice of the optimal control theory in this research is based on its impact on cancer theory. Jarret et al. (2020) explain that the theory has been mainly applied to the growth of the tumor and the development of response actions that include such aspects as chemotherapy, targeted treatments, radiotherapy, immunotherapy, and combinations of these approaches to treatment. The administration of monoclonal antibodies is a type of targeted treatment where the theory can be efficiently applied. Angaroni et al. (2020) also agree that the optimal control theory in pharmacology has been used, especially in determining therapeutic choices such as dosages and schedules that will lead to desired efficacy while ensuring patients also mitigate the costs incurred [8]. Therefore, the theory is effective in demonstrating the approach to dosing mathematically.
However, it is also essential to take note of the limitation of using the theory in the determination of dosing. Jarret et al. (2020) opine that a key limitation of the theory is a challenge in the optimization of response to a tumor, as it may fail to accurately predict the growth of the tumor as well as the distribution of the drugs to the tumor, and the response of the tumor’s cells to the drugs that are administered. Therefore, the application of the theory to the prediction of dosing needs to be based on the progression of the tumor and the response level of the patient to the target drugs. The theory is summarized below (Table 1).
Table 1. Optimal control theory.
| Theory | Key Aspects |
|---|---|
| Optimal Control Theory | Optimizes a solution to a dynamic system. |
| Applies to tumor growth and the development of response mechanisms. | |
| Effective in the development of targeted treatments such as monoclonal antibodies. | |
| May fail to accurately predict the response of tumor cells to drugs administered. |
Variable (body-sized) dosing vs. fixed dosing
MAbs dosing is based on patient-specific factors. The dosing for therapeutics is always aimed at optimizing the therapeutic outcomes for patients by ensuring that adverse effects are minimized and that patients experience positive outcomes based on the dosing regimen used. However, the debate in the oncological field is whether to use variable or fixed dosing regimens for MAbs in oncology. According to Thomas & Balthasar (2019), the biggest challenge in specifically choosing the best possible dosing regimen has been in defining the mechanisms and principles of the inter-subject differences in MAb disposition.
Variable (body-size) dosing
Variable dosing is also commonly referred to as body-size dosing. Most mAbs in oncology are mainly administered using body-size-based schedules as cytotoxic anticancer drugs. This approach follows the variation in one’s body [9]. Hendrikx et al. (2017) put this into context by explaining that variable dosing mainly considers the Body Surface Area (BSA) in m2 for dosing purposes. The concept of dosing based on BSA originates from the narrow therapeutic window of antineoplastic agents [4]. In further exploring this dosing approach, the body weight and the surface area are the most significant clinical covariates used in mAbs pharmacokinetics [10]. The dosing of mAbs is done intuitively, considering that the distribution volume of mAbs is related to the individual's body weight or body surface area. Most importantly, this dosing approach is based on evaluating the variability in the patient’s body and adjusting the dosing schedule to fit such changes in the body, such as an increase or decrease in the patient's weight.
There are various advantages, and disadvantages researchers mention regarding the issue of body size or variable dosing. Manjunath and Pharm take on the general and straightforward approach and, using the example of the American population, end up describing the modalities of various types of dosing. Despite a minor increase in height, the average weight of adults in the United States has risen by 11 kg during the last 50 years [11]. Fixed dosing by multiplying the dosage by the patient's weight is one of the most frequent ways to administer medications. The concept behind body surface area or weight-based dosage is that pharmaceutical pharmacokinetic characteristics rise in line with an individual's physical height. However, dosing pharmaceuticals on a fixed basis implies that drug pharmacokinetic properties do not alter with body mass. Nevertheless, only adults with a narrow range of body sizes are included in the early stages of clinical development. The lack of diversity in this study group does not allow providing an adequate assessment of the relationship between body size and medication clearance. Drugs developed for the US market may not have a dosing schedule based on weight or body surface area. As a result of these dosage strategies, obese patients are more likely to encounter pharmaceutical overexposure or underexposure [11]. This example of the benefits of variable dosing provides essential context on the cancer cases even though it does not necessarily focus on cancer.
In another study, Hendrikx et al. (2017) look at the flip side of the debate, acknowledging that body size dispensation is prevalent in most cases in oncology, with most drugs using the body size schedule rather than the variable dosing schedule. However, they point out that this technique is still debated even though most cytotoxic small molecular anticancer agents are being dosed using the body surface area technique. In the context of cancer, it is a usual practice to inject monoclonal antibodies based on body weight. This is assumed to account for variations in medication distribution and elimination among patients. Only blood plasma and extracellular fluids gain weight in proportion to monoclonal antibody concentrations, higher in plasma. The importance of focusing on such variations is further studied by Eaton and Lyman (2022); they identify the nature of anticancer agents and their dosing. Most anticancer medicines have a strong dose-response relationship and a narrow therapeutic range. If the dose is administered inappropriately, there is a risk of life-threatening toxicity and underdosing, impacting cancer outcomes. Adjuvant treatment for people with curable illnesses such as lymphoma or testicular cancer requires precise dose selection (for example, breast and colon cancer). Individual differences in drug metabolization and excretion make it challenging to determine the proper dosage [12]. Therefore, it is critical to give dosages based on the body size.
Fixed dosing
The simplest definition of fixed dosing is provided by Urgulu and Ozyadin (2014). According to Urgulu and Ozyadin (2014), Fixed-Dose Combination products (FDCs) are developed based on two or more components where the dose for each component fits the standard recommendations. The combination of the active substances is sold as a single product. Fixed-dose combinations are currently widely used for clinical studies and treatment [13]. Understanding fixed dosing based on the differences between the fixed and variable dosing in the oncology context is the base of this study focus. Hendrikx et al. (2017, opine that the volume of distribution changes less than the bodyweight changes due to the distribution volumes of monoclonal antibody changes in underweight and obese people. As a result, underweight patients are given a lower dose than those of normal weight, whereas patients who are obese are given a higher dose based on their weight [4]. When employing fixed dosage, higher doses are delivered to underweight people due to a reduced absolute volume of distribution, whereas doses are lower in obese patients due to a reduced absolute volume of distribution.
Hendrikx et al. (2017) point to whether fixed dosing is the most desirable way or not. There are various positive effects regarding fixed-dose combinations in prescribing. However, these have to be balanced against some of the serious issues raised, including the issues of cost and general irrationality in some cases. Some of the concerns include the possibility of variation of the appropriate dosage of one or more constituents due to the differences in pharmacokinetic profiles and half-life of the constituents. Besides the fact that FDCs may raise the risk of adverse medication responses or drug interactions, FDCs may also fail to adequately account for patients' unique genetic profiles during the development of FDCs [14, 15]. A key consideration in FDCs is pharmacogenetics's role in the components, which may be a primary conduit for removing the drugs of your interest or a critical phase in their start of the action. Aside from the potential for resistance, the pharmacokinetic characteristics of the FDCs' ingredients are also significant in patients with infectious illnesses. Additional considerations for senior patients may have altered the pharmacokinetic and pharmacodynamic profiles of components [16]. Fixed-dose combinations are associated with additional concerns, such as higher costs than individual components. There are difficulties in determining any potential side effects associated with the components, and patients are put at risk of underdose or overdose [16]. Thus, such challenges need to be considered in the course of dosing.
In cases where monotherapy is ineffective, or there are concerns about monotherapy alone, FDCs may offer advantages over prescribing the components separately, such as improved response rates where monotherapy is ineffective, for example, through different mechanisms of action medicines in combination. The proposed FDC minimizes toxicity by potentially counteracting one drug with another.
This section presents the methodology applied to this study. The selection of an appropriate methodology plays a critical role in determining the quality of the data collected and applied to the study. The methodology chapter presents an elaborate comprehension of the adopted research design, the approach to data collection, and the analysis of the collected data.
Research design
The research design applied in this study was the systematic literature review. A systematic review mainly entails identifying, appraising, and synthesizing all the empirical evidence that meets the desired eligibility level in answering a given research question [17]. The researcher had to depend on the available and existing sources to help answer the research question. The massive abundance of studies has made it more appropriate to utilize a systematic review. The selection of this research design was based on its advantages. For instance, systematic reviews were selected because they can limit researcher bias in the study, ensure that reliable and accurate conclusions are made, improve data generalizability and consistency, as well as ensure convenience in terms of time savings on the part of the researcher [18]. However, the limitation of the approach is that it may not always be effective in the provision of accurate details about the topic, especially if the subgroup studied and the research question does not align.
Systematic review process
The systematic review process entails the presentation of the steps undertaken in searching for relevant articles that were applied in this study. The review process illustrates the search strategy, inclusion/exclusion criteria, PRISMA flowchart, and the quality assessment approach.
Search strategy: The first step in the search strategy was the identification of relevant databases that would help in the retrieval of quality articles to be applied in the study. The databases identified were those that easily led to the acquisition of medical journals such as MEDLINE, CIHANL, PubMed, EMBASE, and Cochrane Library. The identification of the databases was followed by the search for needed or relevant articles. The search process for articles entailed keywords, which acted like filters leading to the identification of more specific articles on the topic. The keywords that were input into the databases for the article search were variable dosing, fixed dosing, oncology medication dosing, and monoclonal antibodies. The use of these terms played an instrumental role in leading to the derivation of relevant articles from the databases.
Inclusion/exclusion criteria: The determination of the relevant inclusion/exclusion criteria was also critical to this study because the criteria ensured that only articles, which apply to the study, were used. The first inclusion/exclusion criterion was the type of disease of the participants. In this case, only articles that related to oncology patients were selected. Studies that did not focus on the field of oncology were excluded. Moreover, only studies that focused on the dosing of monoclonal antibodies were included. Those that did not focus on this particular medication were excluded. The study chose only articles published within the last 15 years, while those beyond 15 years of publication were excluded. Lastly, studies included mainly needed to focus on variable or fixed dosing of monoclonal medications. Otherwise, those that did not focus on dosing were excluded.
PRISMA flowchart: The PRISMA flowchart plays an instrumental role in illustrating the search process for the articles and how the articles were screened. The steps that ensured the articles were effectively searched from databases, eligibility identification, and the inclusion/exclusion criteria are presented based PRISMA flow diagram in Figure 1.
Figure 1: PRISMA flowchart.
Quality assessment: The assessment of the quality of the articles collected for the application into the study was helpful, as it ensured that only the best sources were included. Hence, the quality assessment of articles was done using the Critical Appraisal Skills Program tool (CASP). As Long et al. (2020) explained, the CASP tool is an effective quality assessment tool for articles because it facilitates the identification of methodological strengths, limitations, and the research findings in the articles. Consequently, based on the CASP tool, the articles were assessed for their methodological effectiveness and the applicability of their findings to the research questions and objectives set out at the beginning of the study.
Data analysis/synthesis
Analyzing data in systematic reviews leads to the derivation of necessary research outcomes. Data in this systematic research was analyzed using thematic analysis. In this case, relevant themes related to the questions or objectives were gleaned from the articles and used to indepth analyze the problem under investigation. The analysis, in this case, entailed the identification of points of convergence and divergence in the results emerging from previous studies. The findings were compared and differentiated as a part of the data synthesis.
Summary
This chapter considers the methodology used in this study. The systematic literature review was applied in analyzing variable vs. fixed dosing of mAbs in oncology. The use of the systematic review approach was based on the ease of accessing sources and reaching the needed conclusions based on what had been done in previous studies.
The results and discussion are based on the key themes from the articles explored. The organization of the chapter is based on the objectives to ensure the study meets the objectives set out at the beginning.
Objective 1: To examine variable dosing (body-size dosing) of mAbs in oncology, highlighting its pros and cons
Therapeutic Monoclonal Antibodies (mAbs) are typically dosed depending on body mass, believing that this lowers variability in drug exposure among individuals. However, this has not been proven [19]. According to the study's outcomes, this method of dosing was shown to be less than ideal. The use of this dosage method has been brought into question due to a lack of acceptable scientific backing or justification for its use. The starting doses employed in the First-in-Human (FIH) experiment were likely derived from those used in preclinical animal studies. As a result, body-size-based dosing has become increasingly common. Pharmaceutical dose strategies reduce unwanted effects while simultaneously optimizing efficacy in a specific patient population. Finally, judgments on the regimen and clinical dose are influenced by various factors, including the severity of the illness, the features of the patient, compliance, Pharmacoeconomics, and interactions between exposureresponse (efficacy/safety) links. When looking at therapeutic Monoclonal Antibodies that have been approved, the dosing procedures are not clearly described (mAbs). It is believed that when mAbs are administered utilizing the body-size-based dose technique, the variability in drug exposure between individuals is minimized [19]. When body-size-based dosages are used, it is envisaged that this pharmacokinetic variability will significantly impact the variability of treatment response over the entire population as a result.
However, there is no empirical evidence to support the use of body size as a guideline for mAb dose in adults [20]. There is only a 2- fold to 3-fold difference in body mass between adult populations in humans, which does not necessarily suggest that distribution volume and drug-metabolizing capacity are directly proportionate to body size. Even when a statistically significant body size effect on one or more pharmacokinetics is observed in a population pharmacokinetics study, body weight-based dosing is not always justified because pharmacokinetics is rarely linearly related to one's body weight in practice [20]. MAbs must also be taken into consideration. This difference in pharmacokinetics and pharmacodynamic features between monoclonal antibodies and small therapeutic molecules must be considered when developing a dosage paradigm for monoclonal antibodies. Moreover, when it comes to therapeutic outcomes, the pharmacokinetic variability of monoclonal antibodies is usually small (30%-50%) compared to pharmacodynamic variability. Therefore, reducing pharmacokinetic variability may not affect therapeutic outcomes [20]. A bigger therapeutic window than small compounds exists for Monoclonal Antibodies (mAbs) due to their higher selectivity and lower incidence of off-target effects, making them a better choice for treatment [15]. The majority of Monoclonal Antibodies (mAbs) are eliminated by the IgG pathway and the targetdirected drug disposal pathway. MAb affinity and target expression are critical in target-mediated elimination, but neonatal Fc Receptor (FcRn) is important in nonspecific elimination. A multitude of potential confounders, including the levels of target antigen expression, serum protein levels, disease status, and patient demographics such as age, body mass index, and gender, can impact the pharmacokinetics of mAbs [11]. As a result, body size may be responsible for just a small percentage of the interindividual variation in mAb pharmacokinetic characteristics in humans. In other words, due to the broad therapeutic window and the relatively minor contribution of body size to pharmacokinetic and therapeutic result variability, mAb dosage schemes may be more adjustable than other drug administration strategies. Numerous studies have helped researchers better understand the risks and benefits associated with varying antibody dosages and identify feasible alternatives to fixed dosing of antibodies. Ultimately, variable dosing seems to be an outdated approach to the dosing of mAbs, and alternatives should be actively sought, with fixed dosing offering important benefits.
Objective 2: To explore fixed dosing of MAbs in oncology, highlighting its pros and cons
In the current clinical context, almost all the approved monoclonal antibodies in oncology are dosed at a mg per kgâ?ÂÂbased schedule originally developed for trastuzumab [4]. That is, the focus on variable dosing was not necessarily based on proper evidence, especially in adult humans. Instead, it was based on assumptions and what many assumed was the best practice or the following of precedence set without the proper information.
However, it is important to note that there is no clear advantage to be offered by the use of fixed dosing, at least not in all cases. Simulation studies confirmed the effectiveness of fixed dosing for some patients and body-size-based dosing for others [3]. Generally speaking, FixedDose Combination (FDC) therapies offer a means to simplify complex treatment regimens and have several advantages that help patients reach their goals. Generally, fixed-dose formulations usually can give patients some surprising effects compared to only taking any one ingredient in the combinations [21]. Fixed-dose combinations sometimes may provide a synergistic effect in a perfect combination except for the usual addictive effect. Since drugs in formulations from different classes exert their effects based on the individual mechanism with different action sites and action times, fixed-dose combinations in cancer have a potential for modest and long-term action.
Next, a psychological problem must be considered in treating the chronic disease. Since many cancer patients are of advanced age and have poor memory and cannot act easily, the convenience and compliance brought by therapy are especially important. A meta-analysis based on a certain number of databases demonstrated that fixed-dose combinations greatly improved compliance and persistence in cancer treatment. Finally, the cost may also be an obstacle for patients [22]. Combination therapy with fixed-dose may be less costly than the drugs administered separately. Furthermore, combination therapy may reduce the prescribing cost with fewer medications and offer poor patients a lower overall healthcare cost.
tinct categories have been identified as advantageous for fixed dosing. First, there is a generally higher efficacy level than dose monotherapy, at least for some medications. Secondly, there is a lower risk of adverse reactions with the higher dose of monotherapy [3]. Third, a fixed dose offers lower overall costs of medication. Finally, there is an improved concordance in medication. The pros and cons are summarized in Table 2;
Table 2. Pros and cons of fixed dosing.
| Pros | Cons |
|---|---|
| Reduced preparation time. | Potential overdosing of patients. |
| Minimized chances of potential dosing errors. | Increase in drug cost. |
| Alleviation of drug wastage in instances where pooling of preparation is impossible. | |
| Other patients can use the medications when treatment for others is canceled. | |
| There is a reduced inter-subject variability in drug exposure. |
Tartarone et al. (2018) also further characterize potential benefits and drawbacks. Given the fixed nature, they found a general reduction in preparation time for fixed-dose medication. However, this also ran the risk of one possibly being dosed higher than the corresponding personalized dosage. Secondly, they found a decreased chance of errors for fixed dosing and a cost reduction. Cost issues are especially important here, given the high cost of cancer medication. Further drawbacks have outlined the fact that inappropriately manufactured FDCs can reduce effectiveness [23]. While considering fixed dosing, patients must continuously grapple with high costs, which will increase across the coming years, as demonstrated below based on the study (Figure 2) [24].
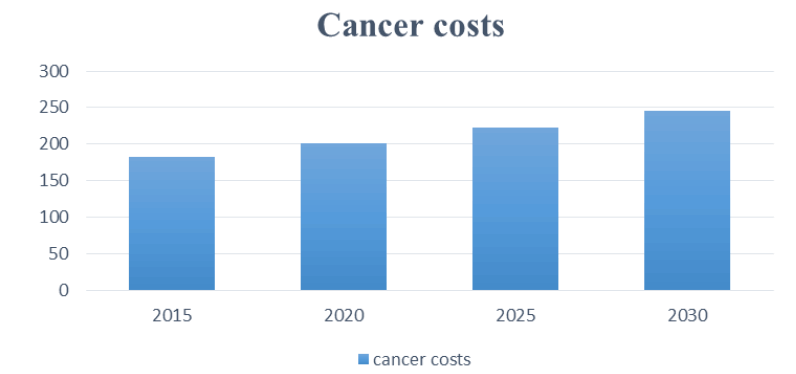
Figure 2: Cancer costs.
A variety of factors cause the high expenses of cancer therapies. Transferring research from the laboratory to patients and completing the appropriate regulatory investigations (such as phase 1, 2, and 3 clinical trials) are both time-consuming and costly [25]. Second, since most cancers are not fully treated, patients usually receive only some agents, but it does not mean that other medications are useless. A third issue is that earlier (now generic) medications were still seen as inferior therapies, even after a monopoly was broken by the launch of “new and improved” versions of an approved drug. A fourth reason for the high expense of treatment is that patients and doctors are willing to pay for even slight improvements in their outcomes due to the seriousness of the cancer diagnosis. Since the systems favor more chemotherapy, legal barriers prevent regulators from considering cost-effectiveness when authorizing new drugs [26]. Based on the Cancer Action Network (2020) explanation, patients tend to bear extremely high costs, as they paid an estimated $5.6 billion out of pocket for cancer treatment in 2018. The chart below illustrates the high costs and distribution paid by different institutions as in Figure 3.
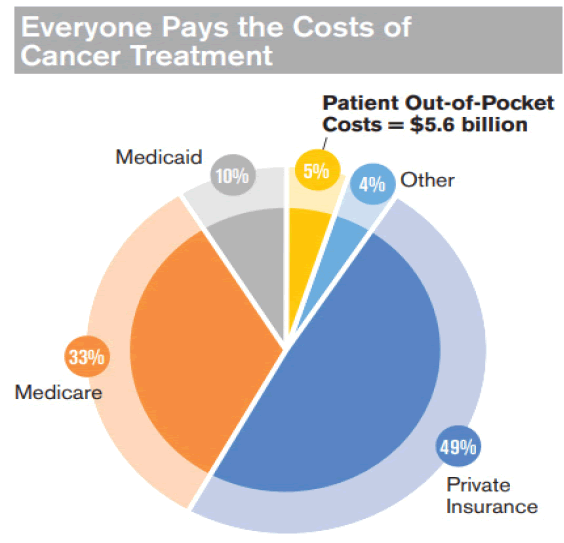
Figure 3: Cancer treatment costs.
Issues of insurance coverage and the extent further complicate the debate [27, 28]. Therefore, the costs are extremely high, and the dosing approach needs to consider such costs. The expense of developing a new drug is high. Preclinical research takes many years and millions of dollars to locate a chemical or manufacture a drug, characterize its mechanism of action, and collect preclinical data. In 2008, pharmaceutical companies invested $50 billion in research and development [29]. Once a medicine is ready for clinical testing, the intricacies of clinical research need costly and time-consuming trial administration, large patient samples, and longer follow-up periods. Each authorized biopharmaceutical costs $1.2 billion to $1.3 billion in financial outlays [29]. Despite a 20-year patient life expectancy, antineoplastic medicines often take 8 years to 10 years to progress from clinical trials to regulatory approval. As a result, the true patent life of a pharmaceutical from its initial commercialization may be brief, often less than ten years. Consequently, only 16% to 19% of medications that go through clinical testing and approval end up on the market when the procedure is completed [29]. Pharmaceutical companies use contract research organizations and contract manufacturing organizations to lower the cost of medicine development. Several factors influence retail medicine costs, including the amount of money spent on research and development, the number of people who will use the drug, the length of the patent, and the estimated return on investment [25]. To illustrate the high costs, the Table 3 below is derived based on the study by Siddiqui & Rajkumar (2018).
Table 3. Cost of selected drugs.
| Generic drug name | Trade name | Cumulative cost for a year $ |
|---|---|---|
| Ipilimumab | Yervoy | 1,20,000 |
| Sipuleucel-T | Provenge | 90,000 |
| Bevacizumab | Avastin | 90,000 |
| Lenalidomide | Revlimid | 90,000 |
| Imatinib mesylate | Gleevec | 70,000 |
Objective 3: To propose an effective dosing approach based on the pros and cons of variable dosing and fixed dosing of mAbs in oncology
Most anticancer medications have a narrow therapeutic range and a strong dose-response association. Small dose changes can cause severe and life-threatening toxicity in some patients and underdosing in others, affecting cancer treatment's long-term efficacy. Proper dosing is crucial in individuals with potentially curable illnesses, such as lymphoma or testicular cancer, and the context of adjuvant treatment [10]. Doses can be difficult to calculate due to individuals’ varying capacities to metabolize and excrete drugs. The most essential pharmacokinetics for drug exposure is the Area Under the Curve (AUC) of plasma concentration x time following a single dosage [12]. Drug level sampling at various points during drug development aids in determining the relationship between drug administration and AUC. Age, gender, weight, height/ weight, concurrent medications, inherited changes in drug-metabolizing enzymes, drug transporters and/or drug targets, and drug clearance are all factors that influence a medication's AUC (which depends on renal and hepatic function). As a result, a wide variety of AUC values result from a single dosage of a specific medication. Most anticancer medications have only attempted to standardize doses based on body size to reduce interindividual variation (weight or Body Surface Area [BSA]).
Prospective and retrospective research aimed at maximizing efficacy while reducing side effects largely defined what dosage should be used in clinical practice for cytotoxic anticancer medicines. Traditionally, animal studies have been used to establish the beginning dose for conventional cytotoxic medicines in phase I clinical trials (the dose that results in lethality in 10% of the treated animals). The first d ose u sed i n human phase I clinical studies has generally been one-tenth of the LD10 [12].
Theoretically and intuitively, larger patients are thought to require more medication to have the same effects since they have a larger volume of distribution and a higher metabolizing capacity. To eliminate inter-individual variance, it is usual to equalize the dosage of anticancer medications depending on Body Surface Area (BSA) and the patient's height and weight. Given the benefits and drawbacks of both constant and variable dosing, fixed dosing looks to be the best option, especially in light of the expenses involved [30-32].
Fixed-Dose Combination Products (FDCs) are medicines that contain two or more active ingredients in fixed proportions in the same formulation. Variable dosing is the opposite of this and has been used as the industry benchmark for a long time. Most mAbs in oncology are mainly administered using body-size-based schedules as cytotoxic anticancer drugs. The approach here is to give dosages as an individual’s body changes. Variable dosing is based on one’s Body Surface Area (BSA) in m2 and is motivated by the narrow therapeutic window of the antineoplastic agents.
Even though variable dosing has often been used as the industry benchmark, there is much debate about its appropriateness, given the benefits fixed dosing offers. Over time, research has revealed that even though there are benefits in some cases in using variable dosing, the overall consensus is that fixed dosing offers important benefits based on the balance of probabilities. After evaluating each option's benefits and drawbacks, the history of use, and appropriateness in the modern context, fixed dosing emerges as a useful alternative. The research concludes that fixed dosing is the better alternative for oncology when it comes to variable versus fixed dosing.
There are several implications for the focus on fixed dosing instead of variable dosing. First, there will be a general level of effectiveness of the medication, both in terms of how it works and in terms of other external factors. For instance, fixed dosing offers such advantages as a reduction in preparation time, a decreased chance of dosing errors, and reduced drug waste. This will make the dosing and administration process much less prone to errors. Second, there are the effects on the cost. Variable dosing increases drug costs. Given the fact that one of the barriers to access to cancer care is the cost of medication, shifting to fixed dosing can be an important step in the right direction.
There are several important possible directions for future study. It is important to note that even though fixed dosing offers distinct advantages, this does not necessarily mean that variable dosing is not appropriate in some cases. Future research should focus on the efficacy of a combined or hybrid approach to dosing that would consider all the important factors in oncology medication.
[Google scholar] [Cross ref]
[Google scholar] [Cross ref]
[Google scholar] [Cross ref]
[Google scholar] [Cross ref]
Citation: Tzenios, N., et al, Variable vs. Fixed Dosing of Monoclonal Antibiotics in Oncology. Med Rep Case Stud. 2022, 07 (10),001-007.
Received: 17-Oct-2022, Manuscript No. MRCS-22-77437; Editor assigned: 19-Oct-2022, Pre QC No. MRCS-22-77437 (PQ); Reviewed: 28-Oct-2022, QC No. MRCS-22- 77437 (Q); Revised: 30-Oct-2022, Manuscript No. MRCS-22-77437 (R); Published: 01-Nov-2022, DOI: 10.4172/2572 5130.22.7(10).1000218
Copyright: ©2022 Tzenios, N et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.